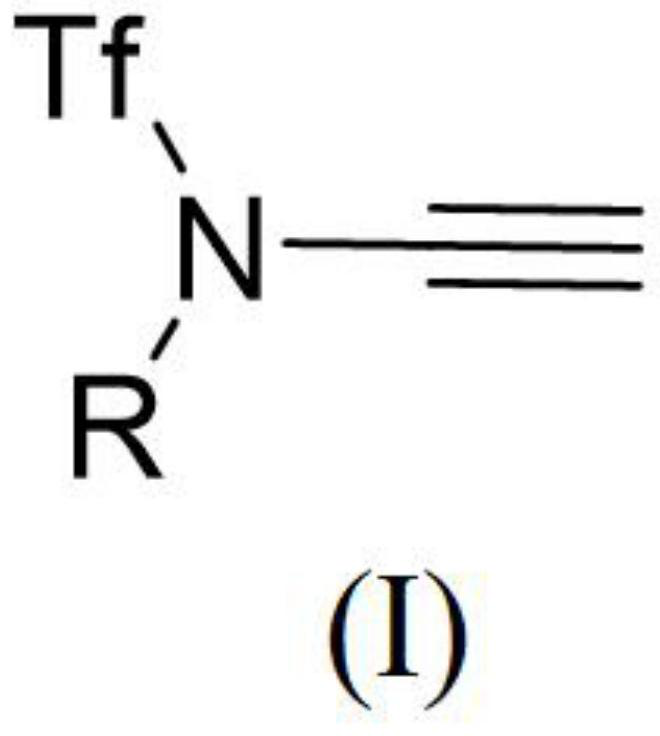
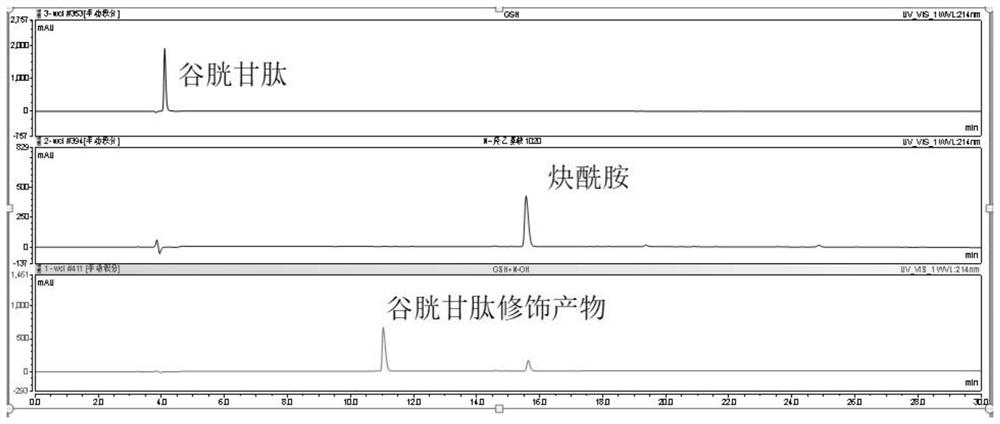
Trifluoromethanesulfonyl alkyne amide compound as well as preparation method and application thereof
A technology of trifluoromethanesulfonyl alkyne amides and amine compounds, which is applied in the field of hydrosulfhydration reaction of alkyne amides, can solve problems such as the instability of addition products, and achieve wide substrate applicability, fast reaction rate, and atom economy high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0088] Preparation of 1,1,1-trifluoromethyl-N-phenylethylsulfonamide:
[0089] Under ice-bath conditions, mix 5mmol phenethylamine and 10mmol triethylamine in dichloromethane solution and stir, slowly add 5mmol trifluoromethanesulfonyl chloride dropwise, add water after the reaction is complete, and extract the aqueous phase with dichloromethane Once, the organic phases were combined, washed once with saturated brine, the organic phase was separated, dried with anhydrous sodium sulfate, and the organic phase was concentrated to obtain 1,1,1-trifluoromethyl-N-phenylethylsulfonamide, light Yellow liquid, yield 92%. The following are the structural formula of the product and the experimental data of nuclear magnetic resonance and mass spectrometry:
[0090]
[0091] 1 HNMR (400MHz, CDCl 3 )δ7.33–7.13(m,5H),4.65(s,1H),3.02(s,2H),2.83(s,2H).
[0092] 13 C NMR (100MHz, CDCl 3 )δ139.4, 129.1, 128.1, 126.7, 121.1, 119.0, 116.8, 114.7, 46.4, 46.3, 463, 46.3, 35.6.
[0093] HR...
preparation Embodiment 2
[0095] Preparation of N-butyl-1,1,1-trifluoromethylsulfonamide:
[0096] Under ice-bath conditions, mix 5mmol n-butylamine and 10mmol triethylamine in dichloromethane solution and stir, slowly add 5mmol trifluoromethanesulfonyl chloride dropwise, add water after the reaction is complete, and extract the aqueous phase with dichloromethane Once, the organic phases were combined, washed once with saturated brine, the organic phase was separated, dried with anhydrous sodium sulfate, and the organic phase was concentrated to obtain N-butyl-1,1,1-trifluoromethylsulfonamide, light yellow Liquid, 92% yield. The following are the structural formula of the product and the experimental data of nuclear magnetic resonance and mass spectrometry:
[0097]
[0098] 1 H NMR (400MHz, CDCl 3 )δ4.47(s,1H),2.87(s,2H),1.51(s,2H),1.30(s,2H),0.90(s,3H).
[0099] 13 C NMR (100MHz, CDCl 3 )δ121.11, 118.97, 116.83, 114.68, 47.27, 47.24, 47.21, 47.18, 31.07, 20.45, 14.00.
[0100] HRMS(ESI)m / z ...
preparation Embodiment 3
[0102] Preparation of tert-butyl (2-((trifluoromethyl)sulfonyl)ethyl)carbamate:
[0103] Under ice-bath conditions, mix 5 mmol n-butyl (2-aminoethyl carbamate) and 10 mmol triethylamine in dichloromethane solution and stir, slowly add 5 mmol trifluoromethanesulfonyl chloride dropwise, and the reaction is complete After adding water, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, washed once with saturated brine, the organic phase was separated, dried with anhydrous sodium sulfate, and the organic phase was concentrated to obtain tert-butyl (2-((three Fluoromethyl)sulfonyl)ethyl)carbamate, light yellow liquid, yield 95%. The following are the structural formula of the product and the experimental data of nuclear magnetic resonance and mass spectrometry:
[0104]
[0105] 1 H NMR (400MHz, CDCl 3)δ8.52(s,1H),4.44(s,1H),3.42(s,2H),2.86(s,2H),1.42(s,9H).
[0106] 13 C NMR (100MHz, CDCl 3 )δ158.57, 121.11, 118.97, 116.83, 114.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com