Construction method and application of novel gene modification enhanced NY-ESO-1 specific TCR-T model
A NY-ESO-1, TCR-T technology, applied in genomics, instruments, biological systems, etc., can solve the problems of multi-cytokine, chemokine efficiency and functional differences, and achieve strong chemotactic migration ability, Produce controllable and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
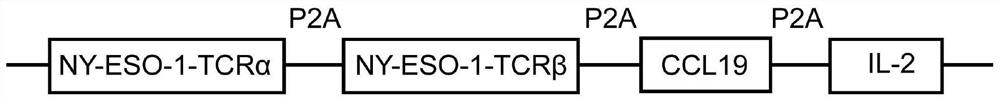
[0030] Construction of recombinant expression vector pCDH-CMV-MCS-EF1-Puro-TCR(NY-ESO-1)-CCL19-IL-2:
[0031] The insert of pCDH-CMV-MCS-EF1-Puro-TCR(NY-ESO-1)-CCL19-IL-2 includes encoding TCR α chain, β chain that specifically recognizes NY-ESO-1, expressing CCL19 and IL-2 The nucleic acid fragments are linked by P2A sequences, such as figure 1 As shown, the nucleic acid sequence of the insert is as figure 2 shown.
[0032] The construction process includes: adding XbaI and BamHI restriction enzyme cleavage site sequences at both ends of the TCR(NY-ESO-1)-CCL19-IL-2 insert. The nucleic acid fragment was double digested with the lentiviral backbone vector pCDH-CMV-MCS-EF1-Puro with restriction enzymes XbaI and BamHI, and the digested product was recovered by gel cutting and then ligated with T4 ligase overnight. The competent E. coli was transformed, coated on the plate, and the next day, the single clone was picked for double digestion and sequencing identification, and t...
Embodiment 2
[0034] Enhanced TCR-T specificity test:
[0035]T2 cells were used as antigen-loaded cells, loaded with two NY-ESO-1 polypeptides (20ug / ml each) and incubated for 3h, washed three times with PBS to remove free polypeptides.
[0036] Adjust the enhanced TCR-T cell density to 1X10 5 / ml, with 1X10 5 After co-cultivation with T2 / ml for 2h, 10ug / ml Breiflid A was added to incubate overnight, and T2 cells without polypeptide loaded were used as negative control. After that, follow-up IFN-γ flow detection was performed, and the proportion of CD3 + IFN-γ + positive cells in CD3 + T cells was analyzed by flow cytometry.
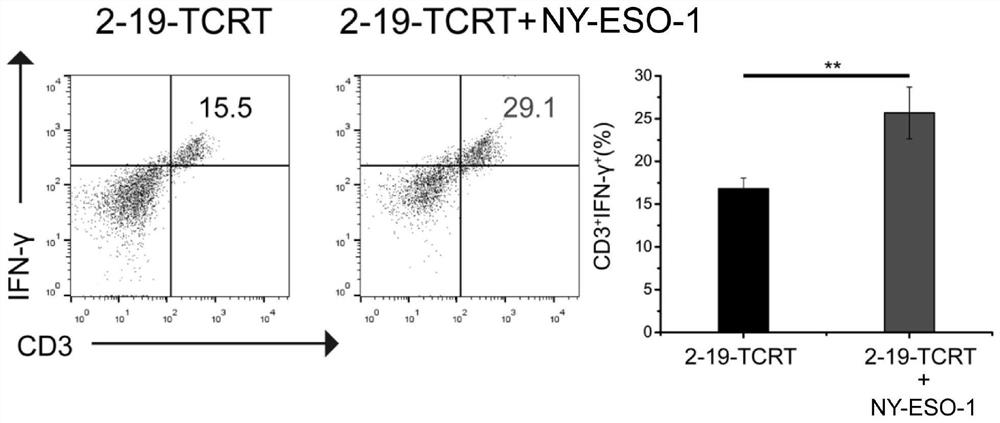
[0037] image 3 T cells (2-19-TCR-T) infected with pCDH-CMV-MCS-EF1-Puro-TCR(NY-ESO-1)-CCL19-IL-2 recombinant vector were stimulated with NY-ESO-1 antigen Streaming results.
[0038] The test results showed that after T2-loaded NY-ESO-1 antigen stimulation, the 2-19-TCR-T immune response was more significantly enhanced than the control, indicating that it has an...
Embodiment 3
[0040] Quantitative detection of CCL19 produced by enhanced TCR-T:
[0041] Take TCR-T and 2-19-TCR-T culture supernatants respectively, and store them at minus 20 degrees Celsius for testing.
[0042] Standard samples were added, and the standard samples with a concentration of 480 pg / ml in the mother liquor were half-diluted with the sample diluent to obtain a series of standard solutions with concentrations of 480, 240, 10, 60, 30, and 0 pg / ml, respectively.
[0043] Set standard wells and sample wells, and add 50ul of different concentrations of standard to each standard well.
[0044] Set up blank wells and sample wells respectively, add 40ul of sample diluent to the wells of the microtiter plate to be tested, then add 10ul of the sample to be tested, add the sample to the bottom of the well, and shake gently to mix.
[0045] Add 100ul of enzyme labeling reagent to each well, except for blank wells.
[0046] Incubate at 37°C for 60 min after sealing with a sealing film....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com