Patents
Literature
35 results about "Neoplasm DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
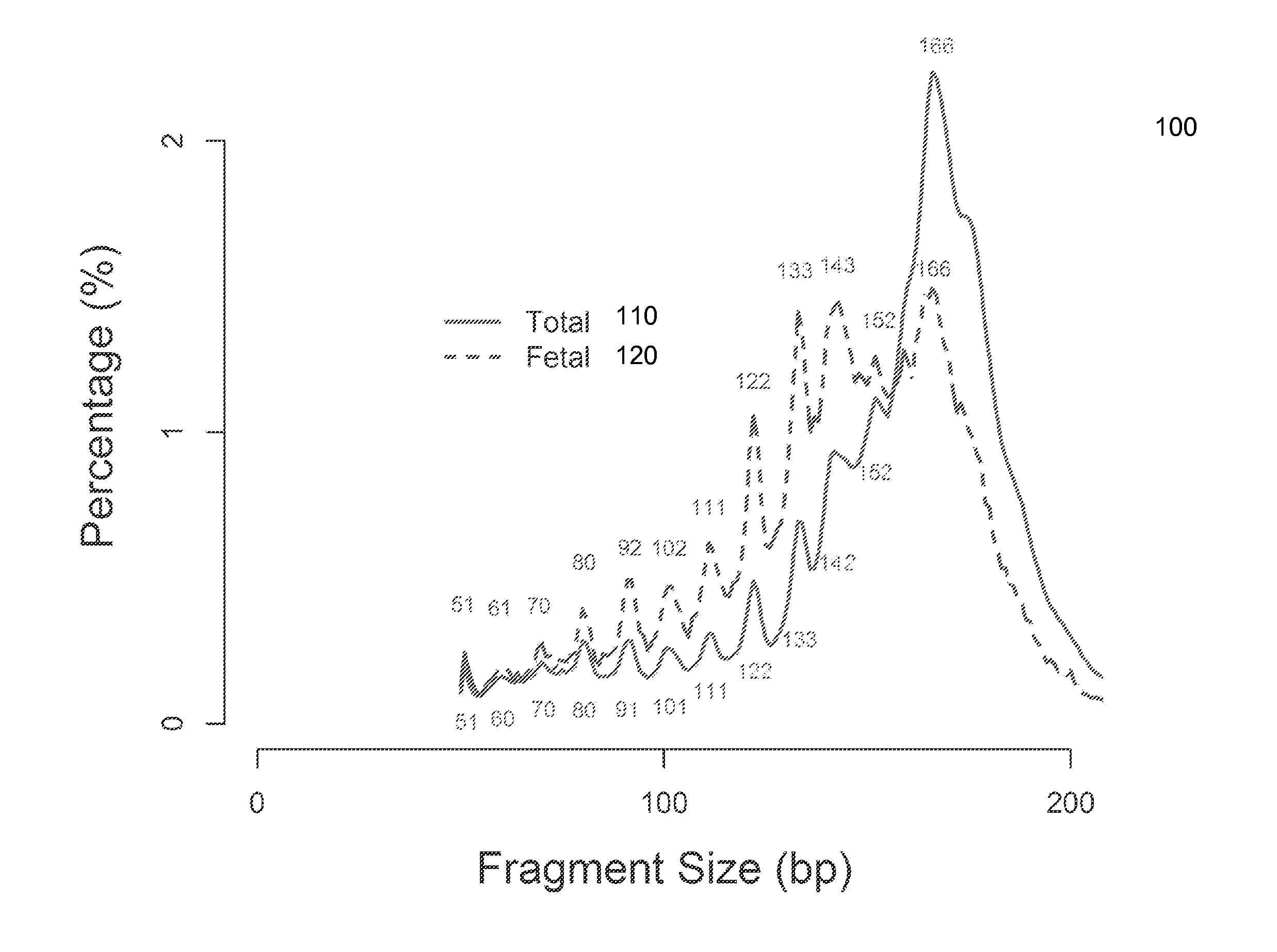
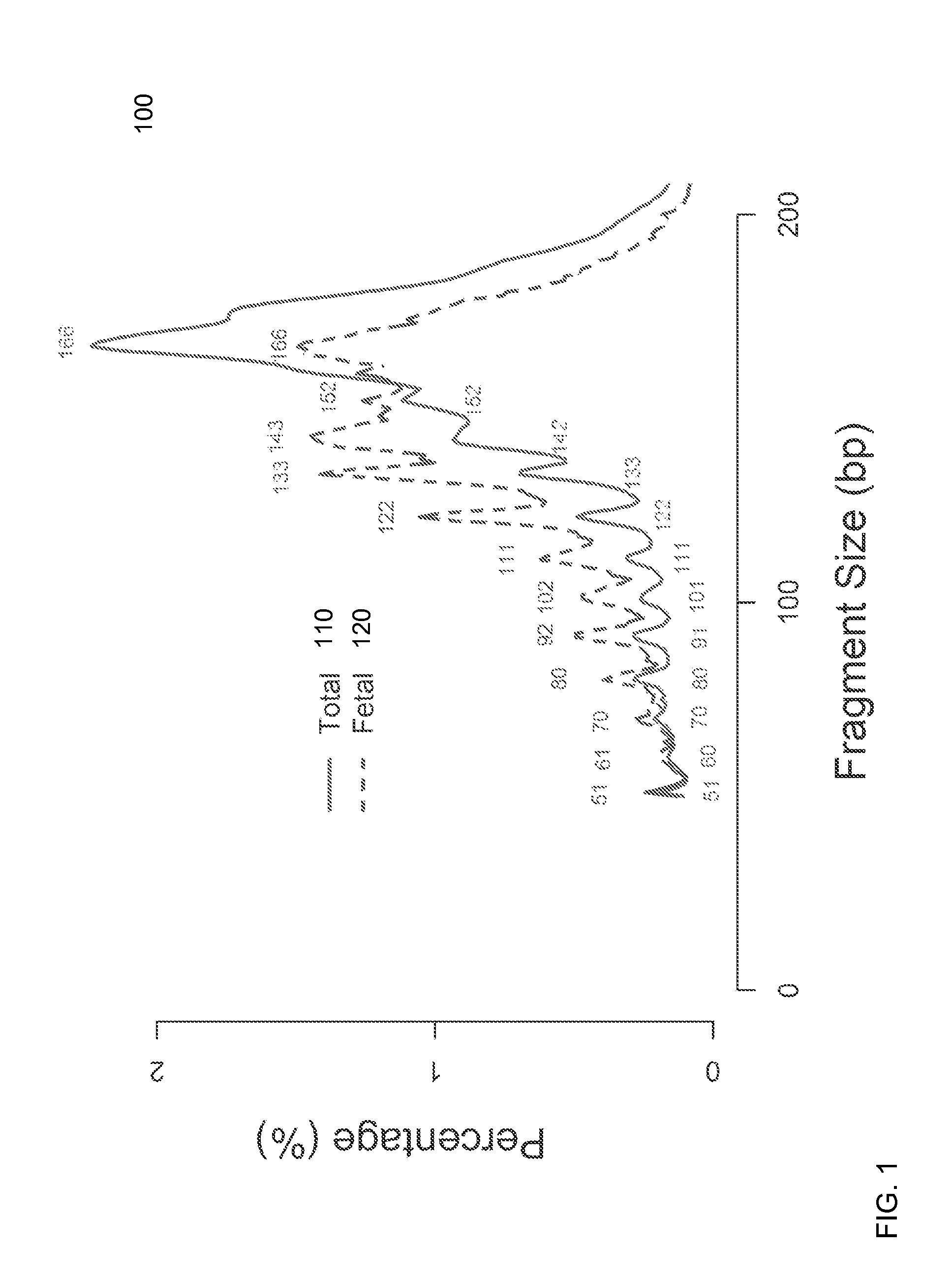
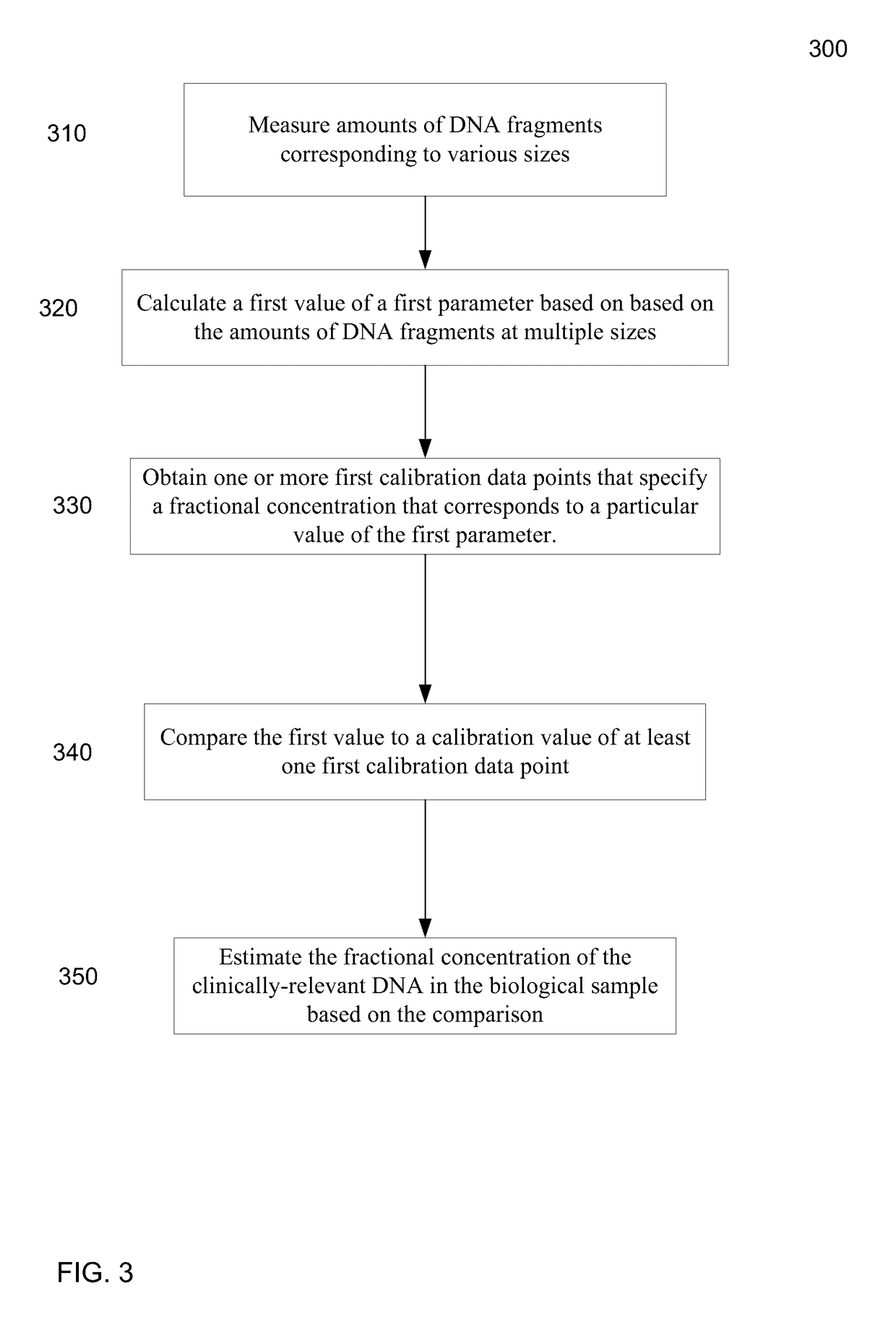
Size-based analysis of fetal DNA fraction in maternal plasma
ActiveUS20130237431A1Health-index calculationMicrobiological testing/measurementAbnormal tissue growthBlood plasma
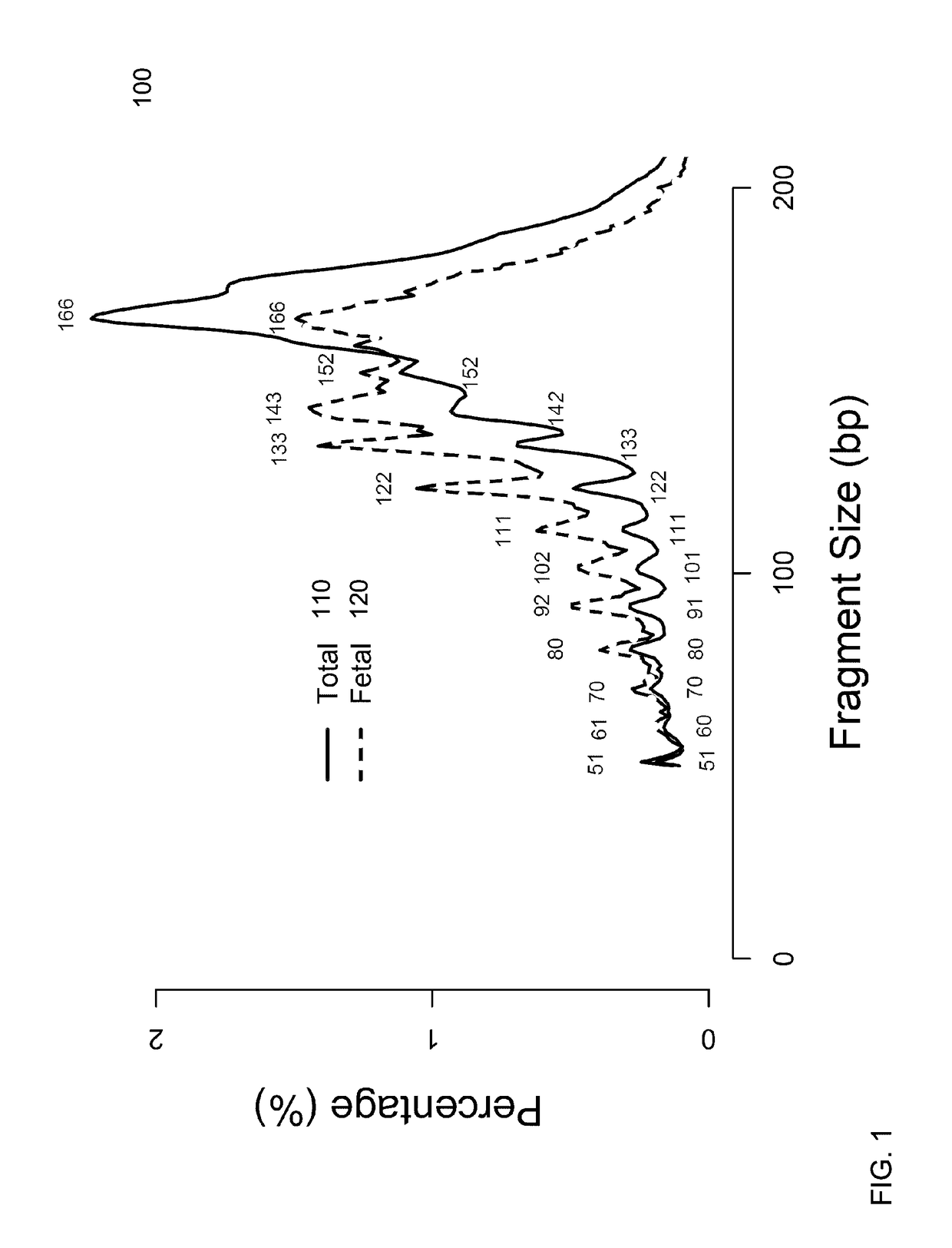
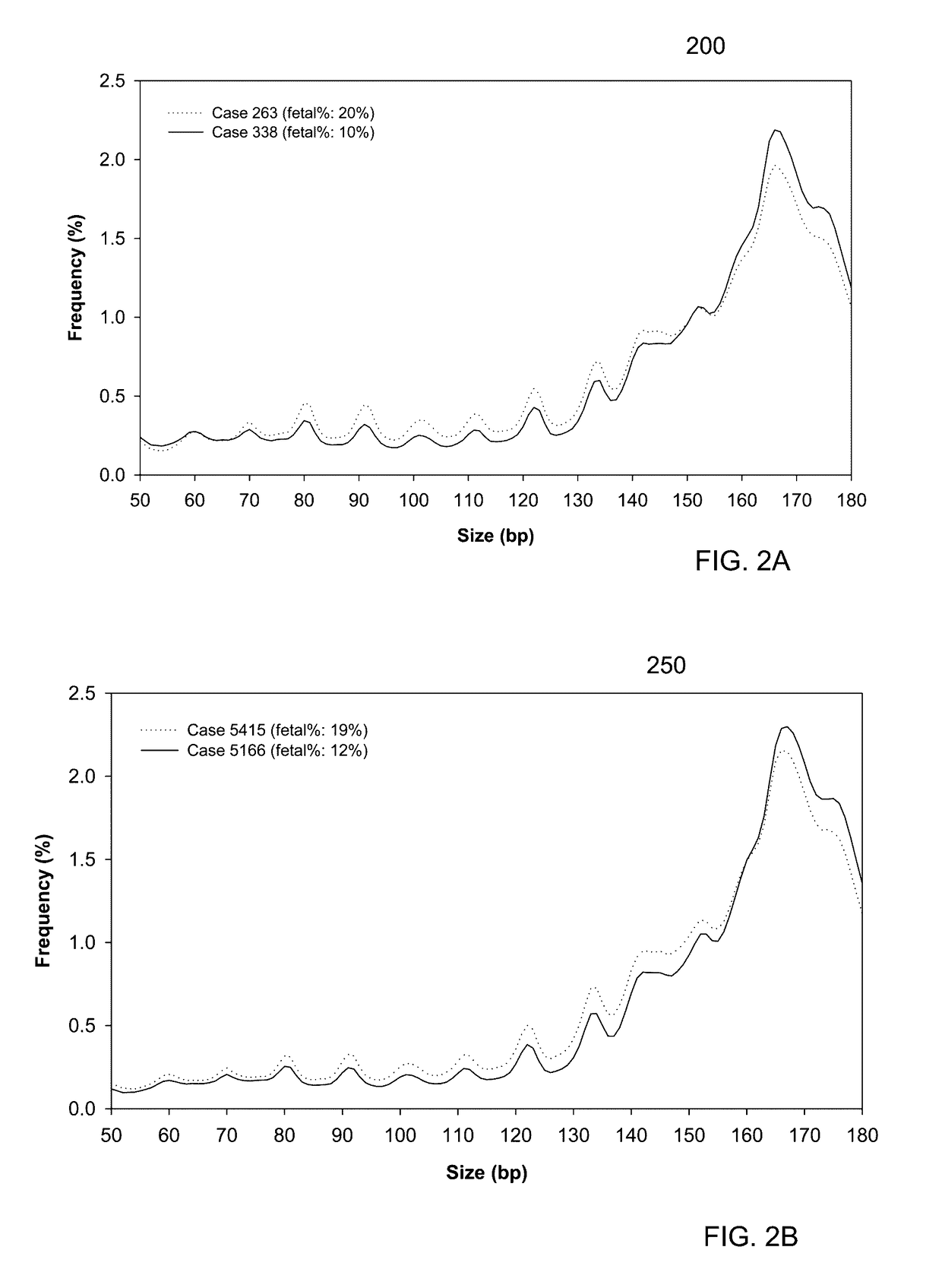
A fractional concentration of clinically-relevant DNA in a mixture of DNA from a biological sample is determined based on amounts of DNA fragments at multiple sizes. For example, the fractional concentration of fetal DNA in maternal plasma or tumor DNA in a patient's plasma can be determined. The size of DNA fragments in a sample is shown to be correlated with a proportion of fetal DNA and a proportion of tumor DNA, respectively. Calibration data points (e.g., as a calibration function) indicate a correspondence between values of a size parameter and the fractional concentration of the clinically-relevant DNA. For a given sample, a first value of a size parameter can be determined from the sizes of DNA fragments in a sample. A comparison of the first value to the calibration data points can provide the estimate of the fractional concentration of the clinically-relevant DNA.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Specific amplification of tumor specific DNA sequences
InactiveUS20100240549A1Useful in detectionNucleotide librariesMicrobiological testing/measurementCancer detectionTumor specific
The present invention provides methods for cancer detection and diagnosis. The present invention provides a method of selectively amplifying hypomethylated tumor DNA sequences derived from a subject for detection of cancer. This method utilizes differential methylation to allow for the selective amplification of tumor specific sequences from DNA mixtures that contain a high proportion of normal host DNA. The invention also provides methods of using the amplified tumor DNA sequences for evaluation of methylation.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
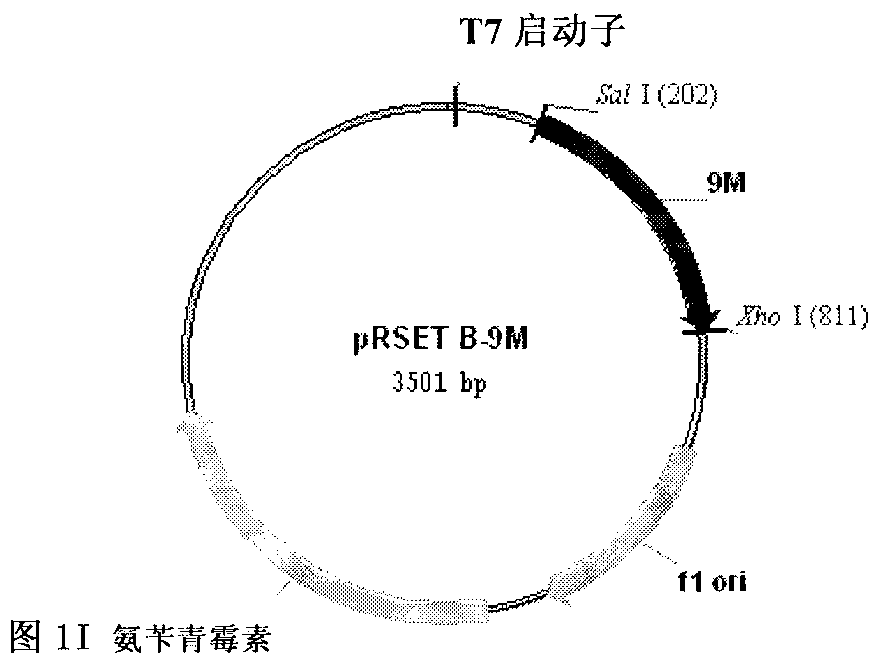
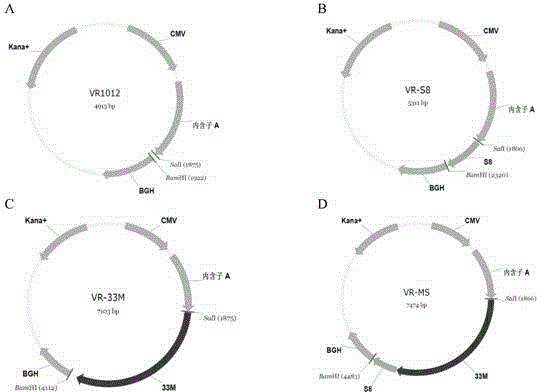
Tumor DNA vaccine taking mucin 1 and survivin as targets and viral vector vaccine
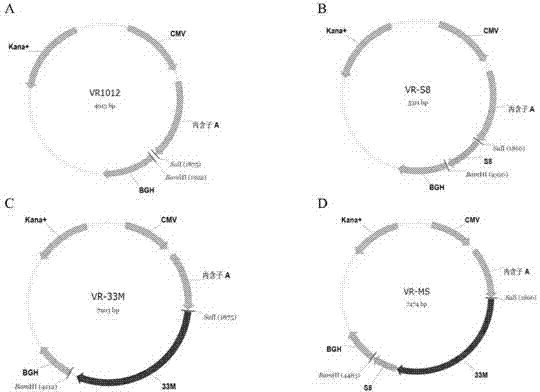
ActiveCN102086453AImprove immunityImprove tumor inhibition rateGenetic material ingredientsMicroorganism based processesAntineoplastic VaccineNeoplasm DNA
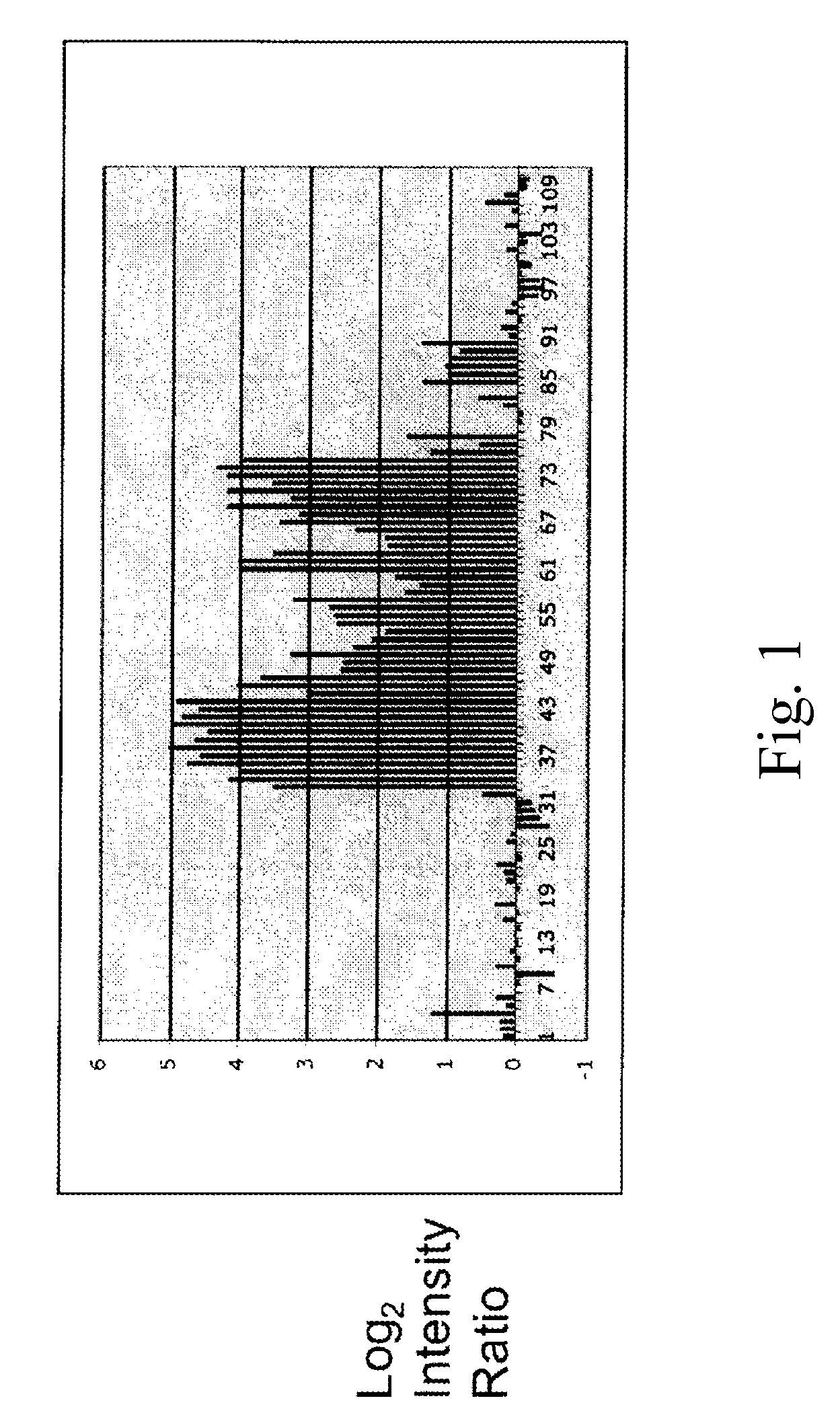
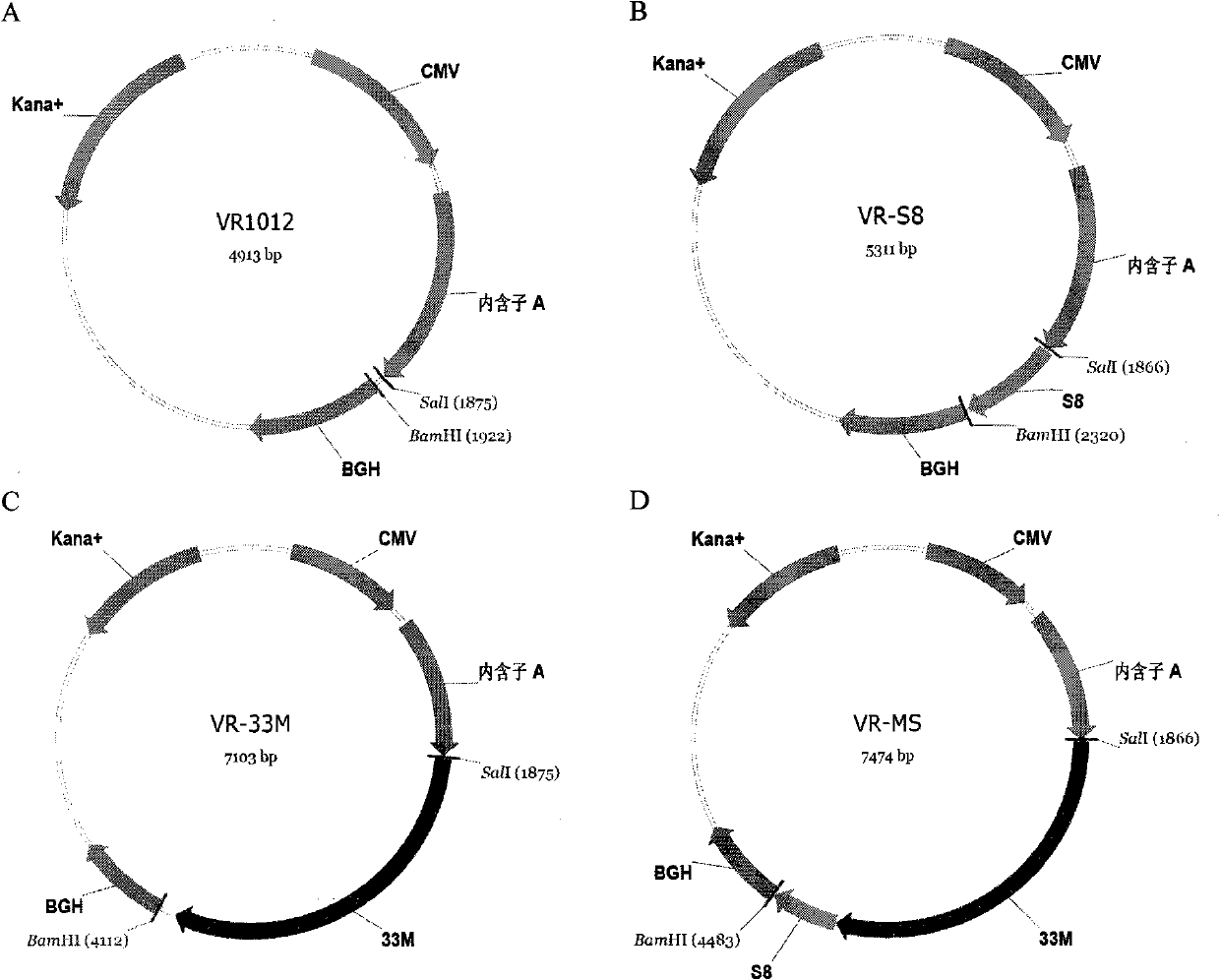
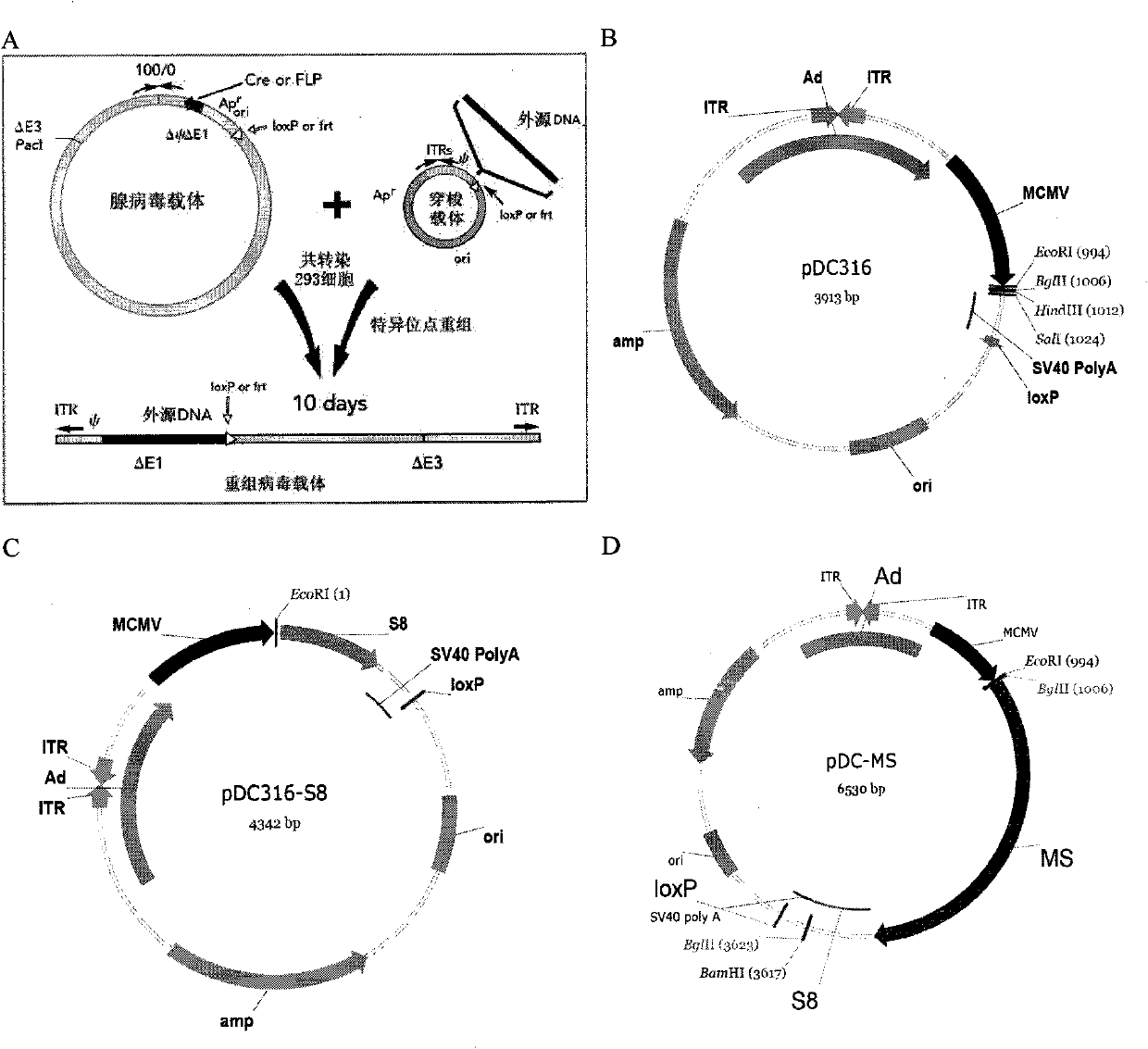
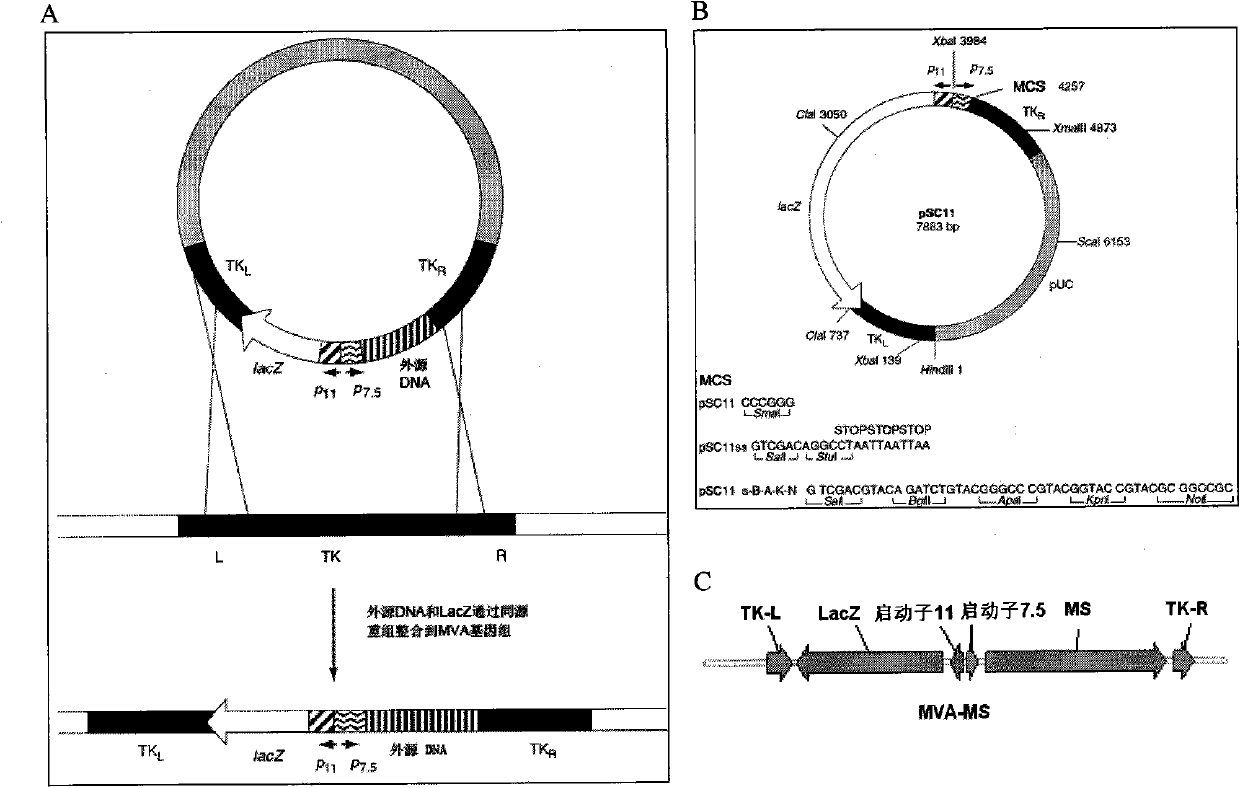
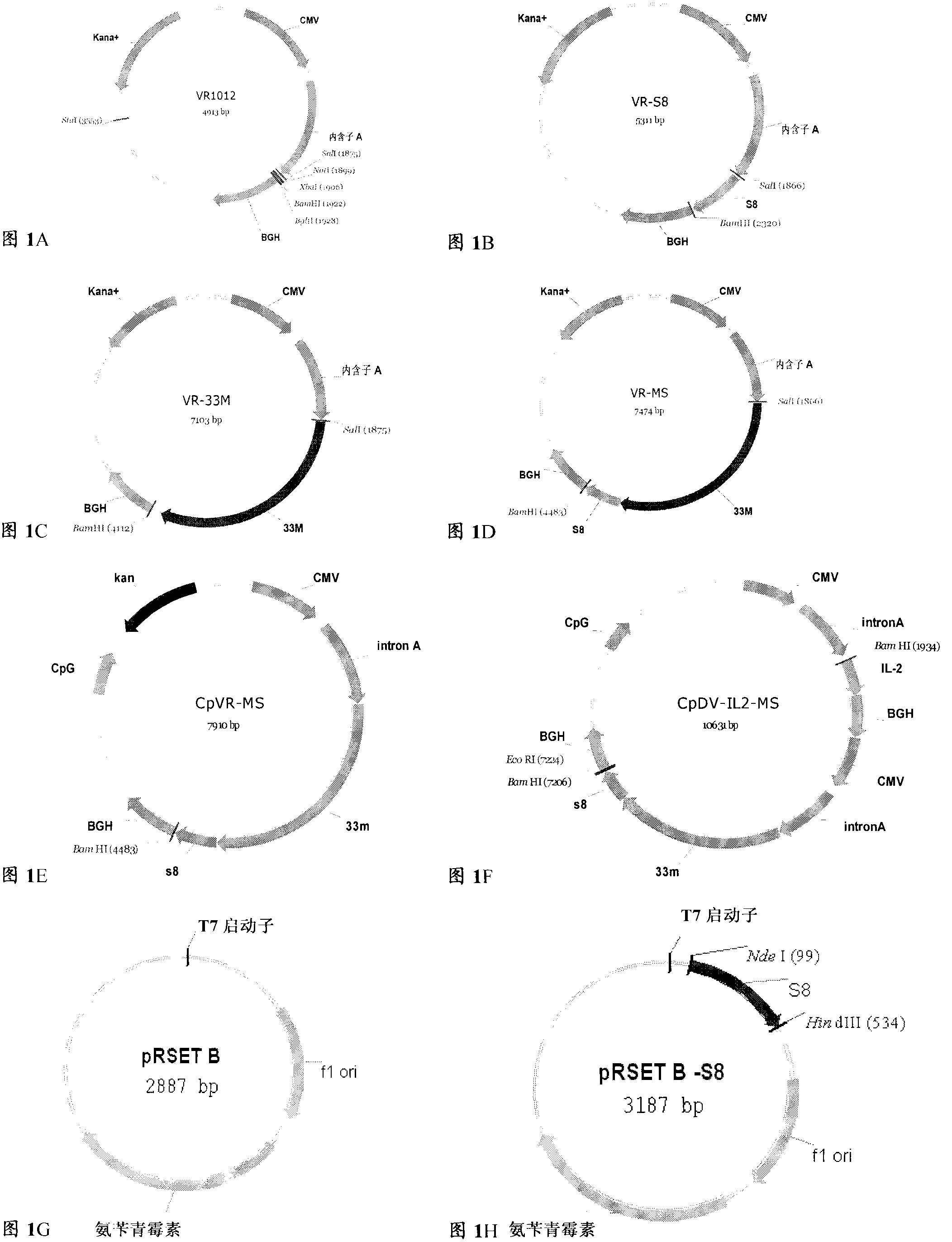
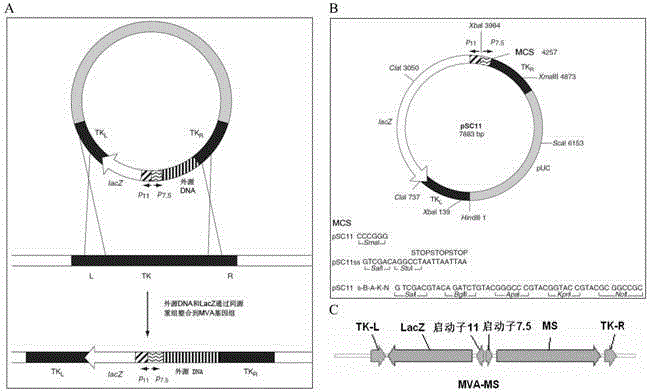
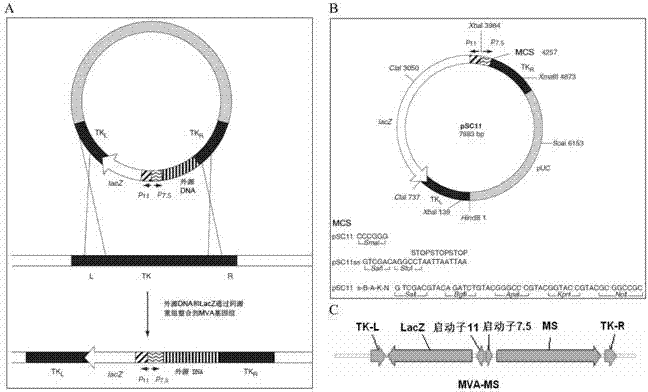
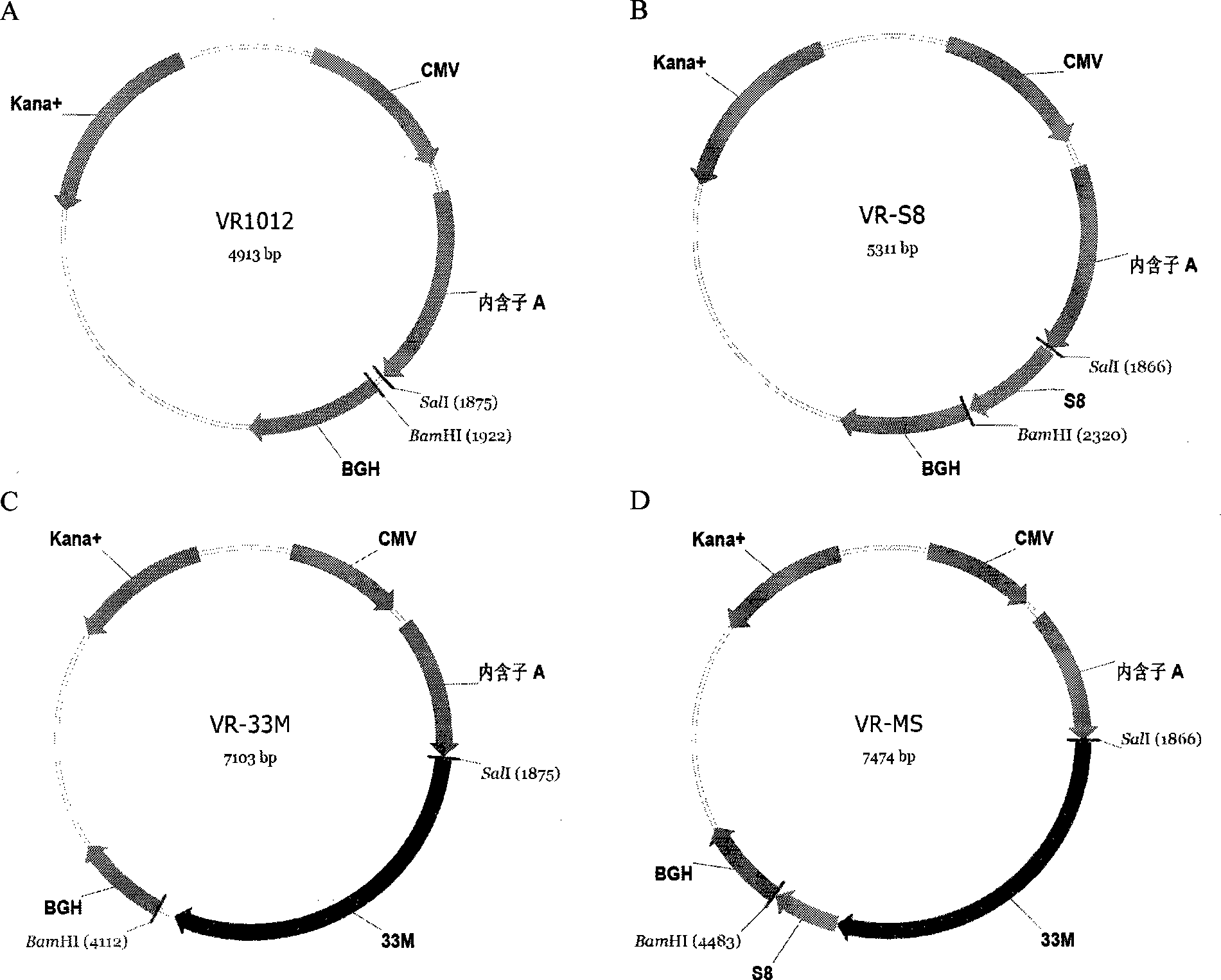
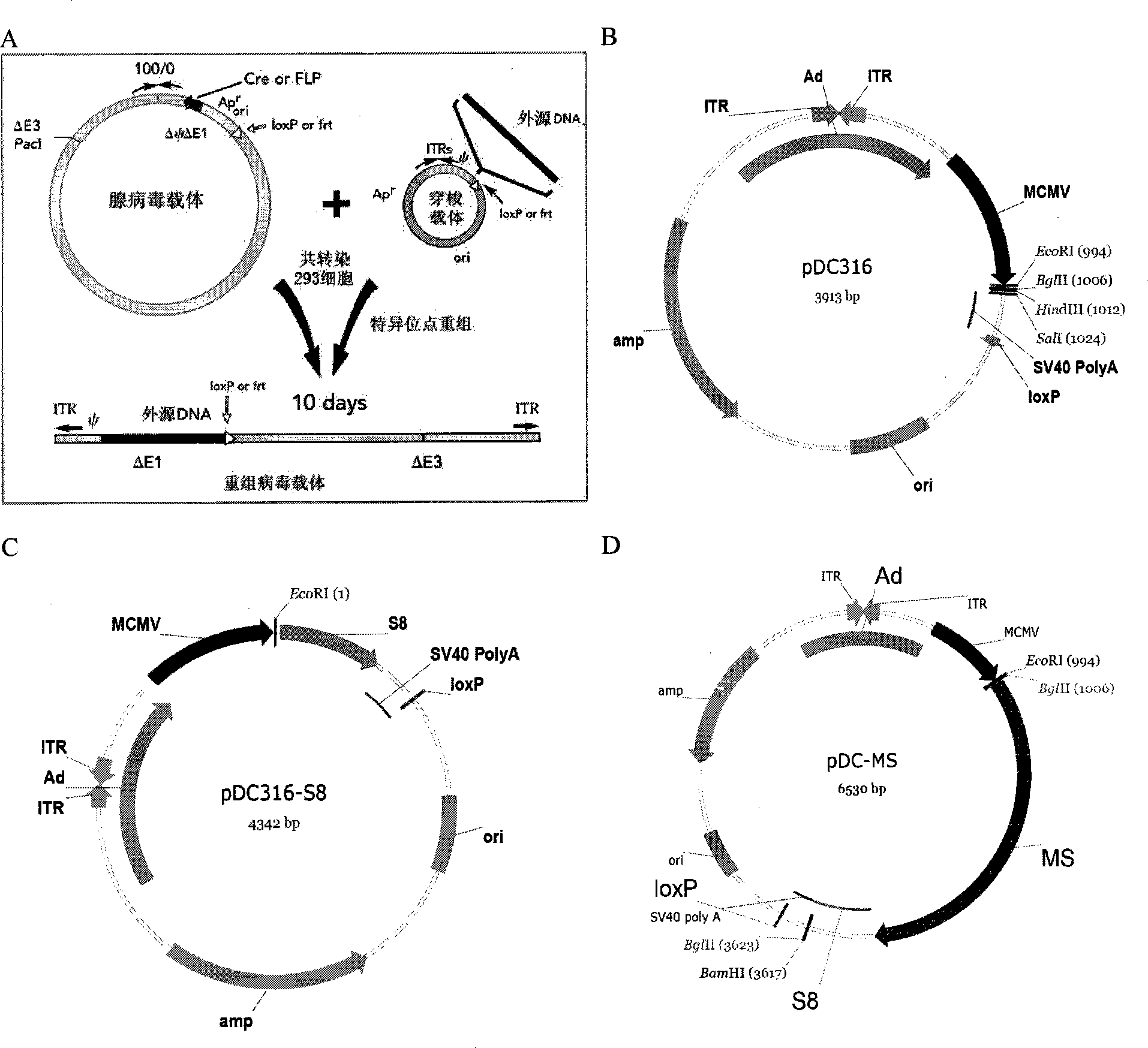
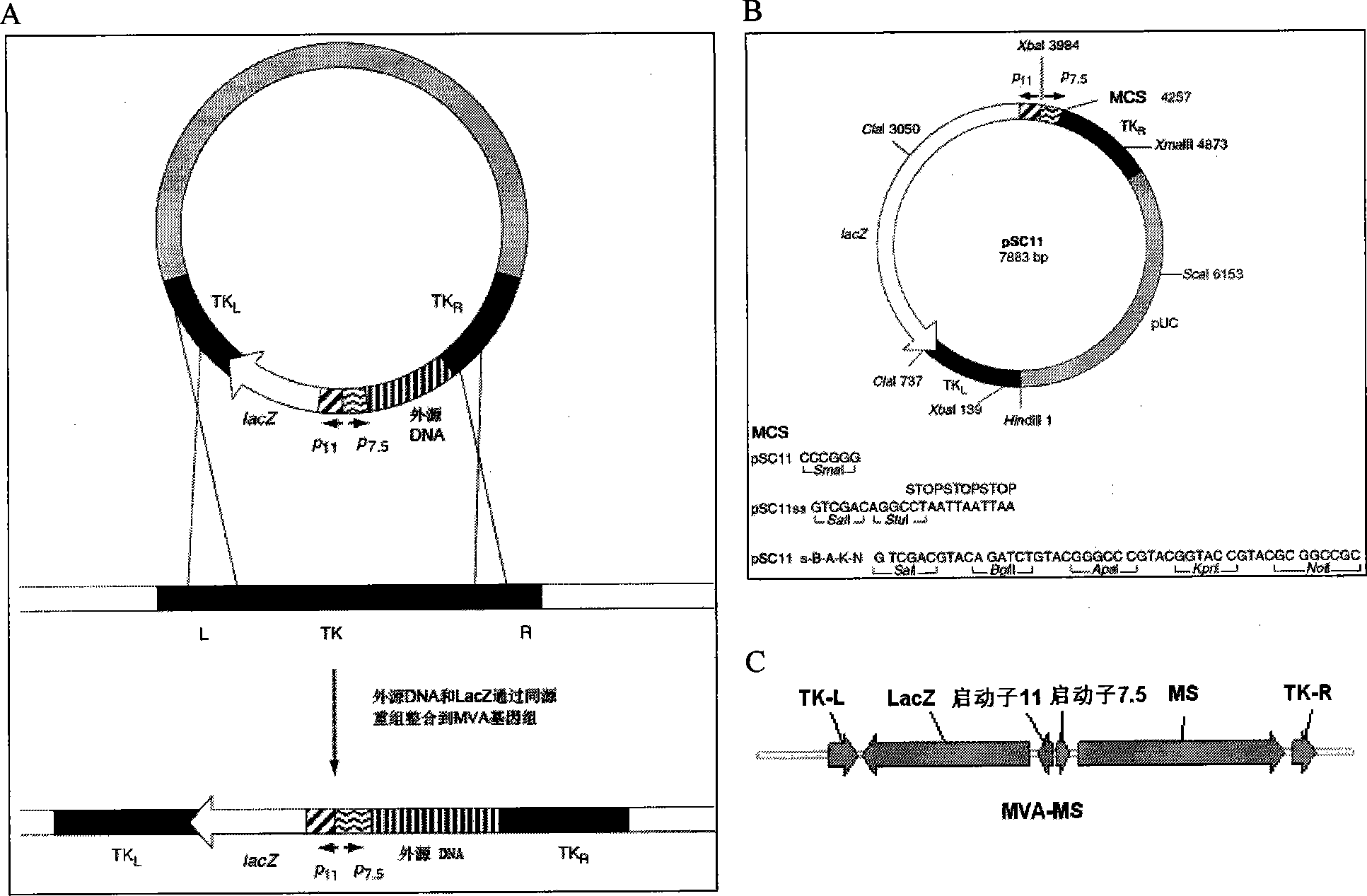
The invention discloses a tumor deoxyribonucleic acid (DNA) vaccine taking mucin 1 and survivin as targets and a viral vector vaccine. The invention relates to the field of tumor DNA vaccines and viral vector vaccines, in particular to recombinant DNA vector VR-S8 and VR-MS, recombinant adenovirus Ad-S8 and Ad-MS, and recombinant poxvirus MVA-MS vaccines, and application of the optimum combination of immune ways between DNA vaccines and viral vector vaccines and between the viral vector vaccines in preparing antitumor vaccines.
Owner:CHANGCHUN BCHT BIOTECH +1
Application of sequence-optimized secreted form FAPalpha-based tumor DNA vaccine
PendingCN110205335AImprove securityCapable of secreting and expressingPolypeptide with localisation/targeting motifCancer antigen ingredientsProtein targetTumor therapy
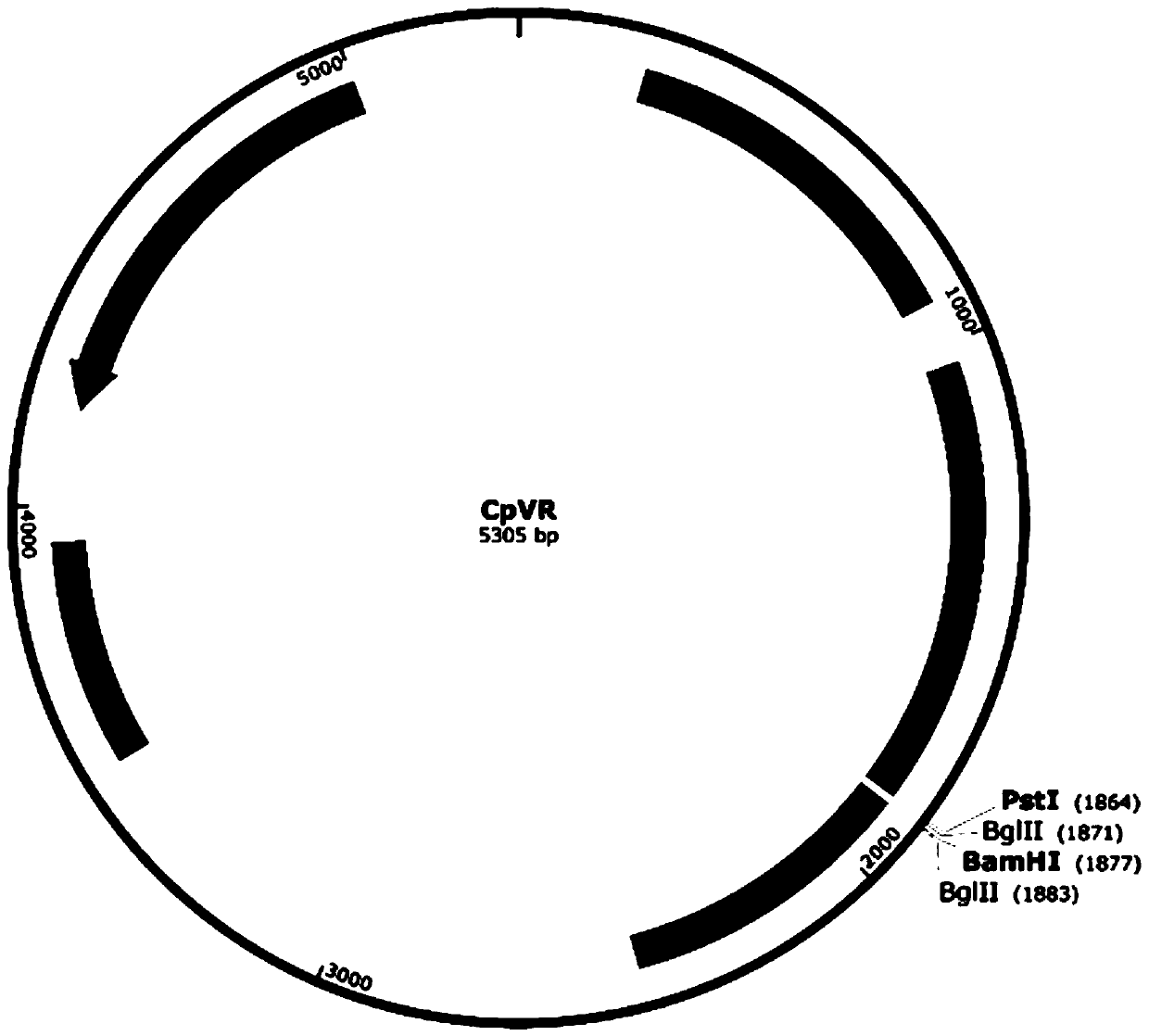
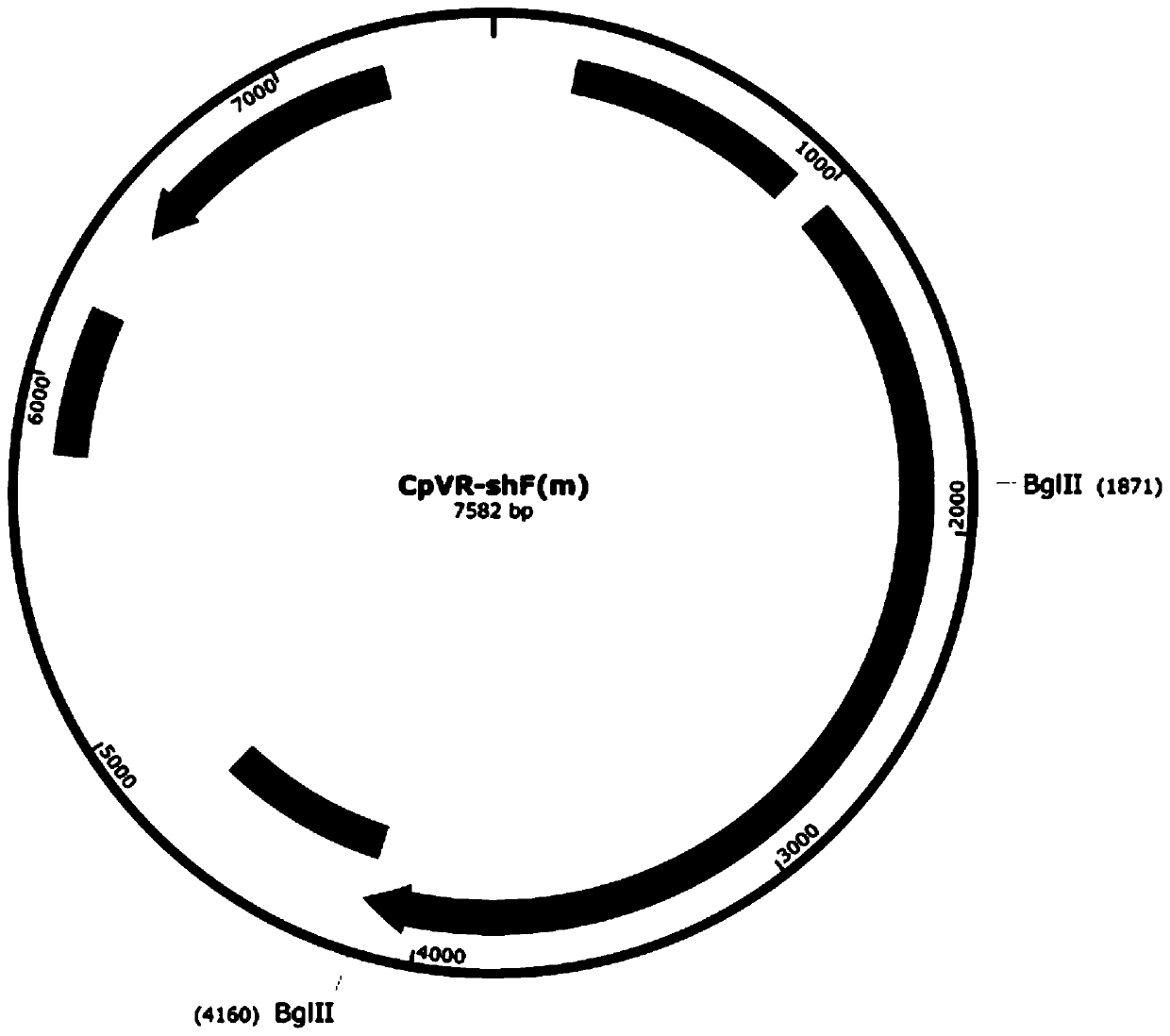
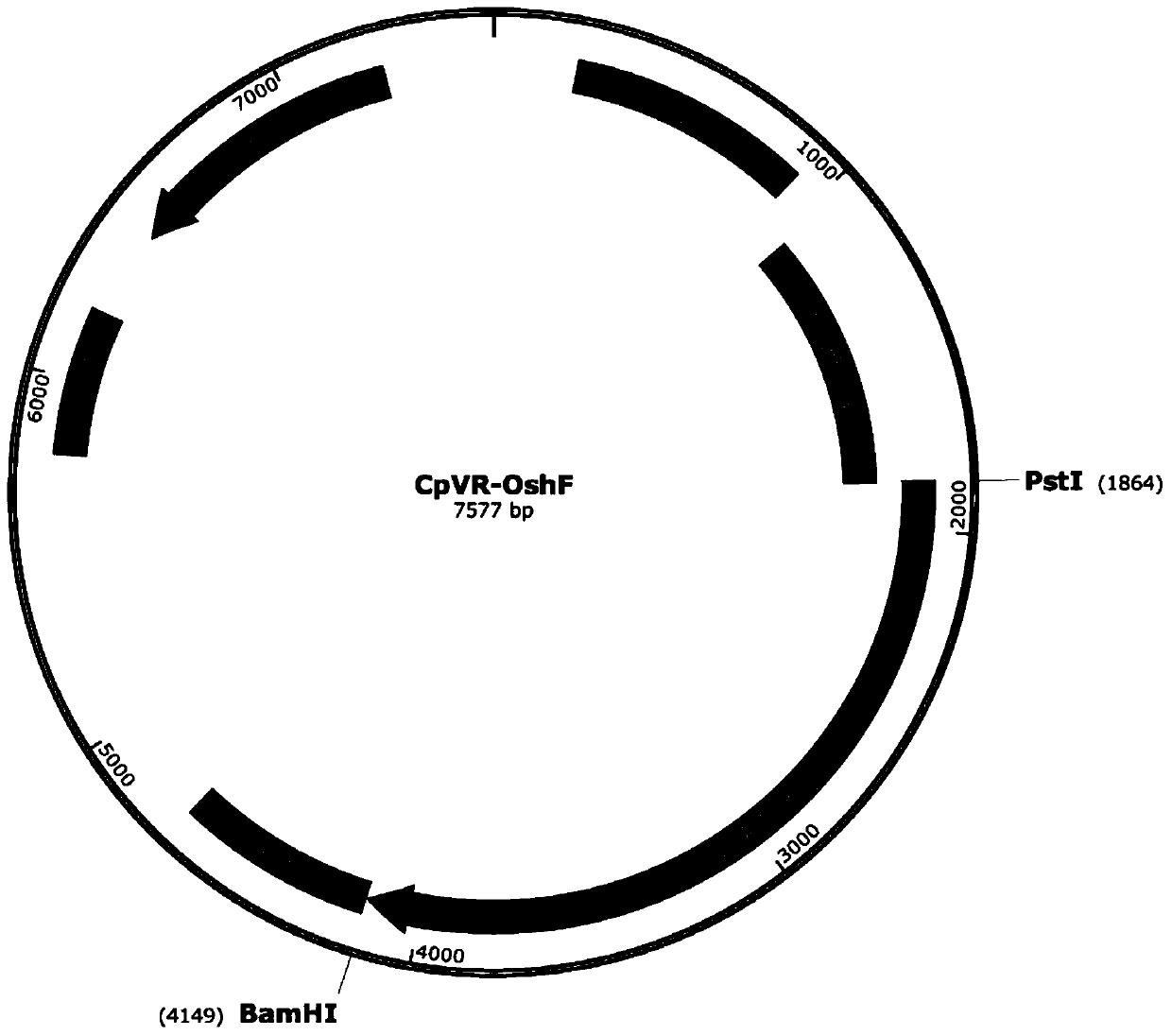
The invention relates to an application of a sequence-optimized secreted form FAPalpha-based tumor DNA vaccine, which belongs to the field of a tumor DNA vaccine. The invention relates to a FAPalpha-derived secreted form FAPalpha extracellular segment sequence shF(m) and its codon-optimized sequence OshF(m) and an application of a DNA vaccine constructed based on two sequences for preparing a tumor therapeutic vaccine. The application determines, by immunogenicity experiments and anti-tumor experiments, that the above DNA vaccine has stronger antitumor activity than a DNA vaccine having the original FAPalpha sequence. the sequence OshF(m) has only 76% homology with the original sequence, and can better express the target protein, and the application has a better application prospect in thepreparation of various tumor vaccines based on FAPalpha.
Owner:JILIN UNIV
Tumor genetically-engineered vaccine utilizing mucin-1 and survivin as target sites
ActiveCN102732543AImprove immunityImprove securityPeptide/protein ingredientsGenetic material ingredientsViral VaccineA-DNA
The invention relates to fields of tumor DNA vaccines, viral vector vaccines and protein vaccines and especially relates to a tumor genetically-engineered vaccine utilizing mucin-1 and survivin as target sites. The tumor genetically-engineered vaccine comprises recombinant DNA vectors of CpVR-MS and CpDV-IL2-MS. The invention also relates to a use of recombinant protein vaccines of S8 and 9M, a DNA vaccine, a viral vaccine, a protein vaccine and an optimized combination of immune methods of the DNA vaccine, the viral vaccine and the protein vaccine in preparation of an anti-tumor vaccine.
Owner:CHANGCHUN BCHT BIOTECH +1
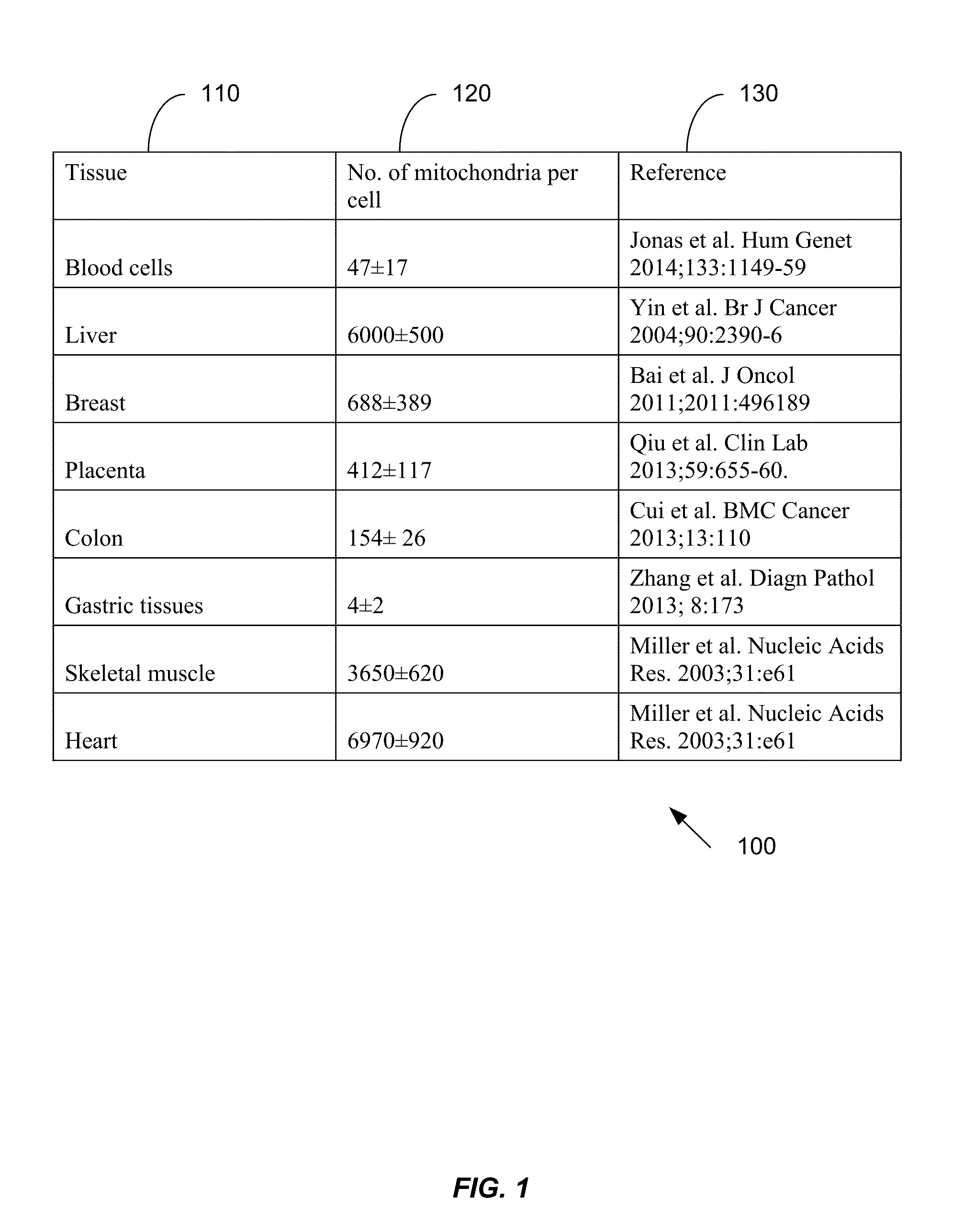
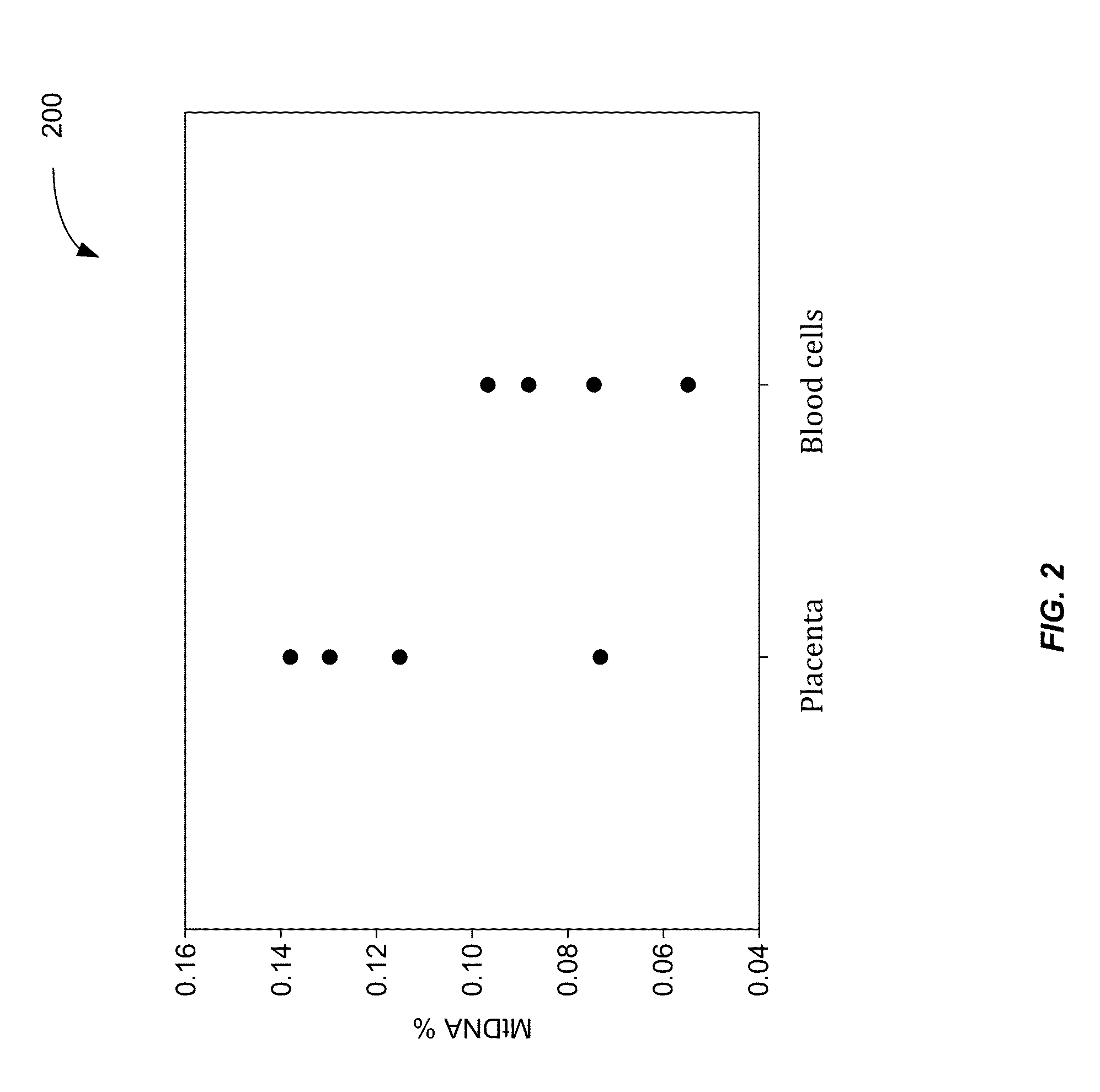
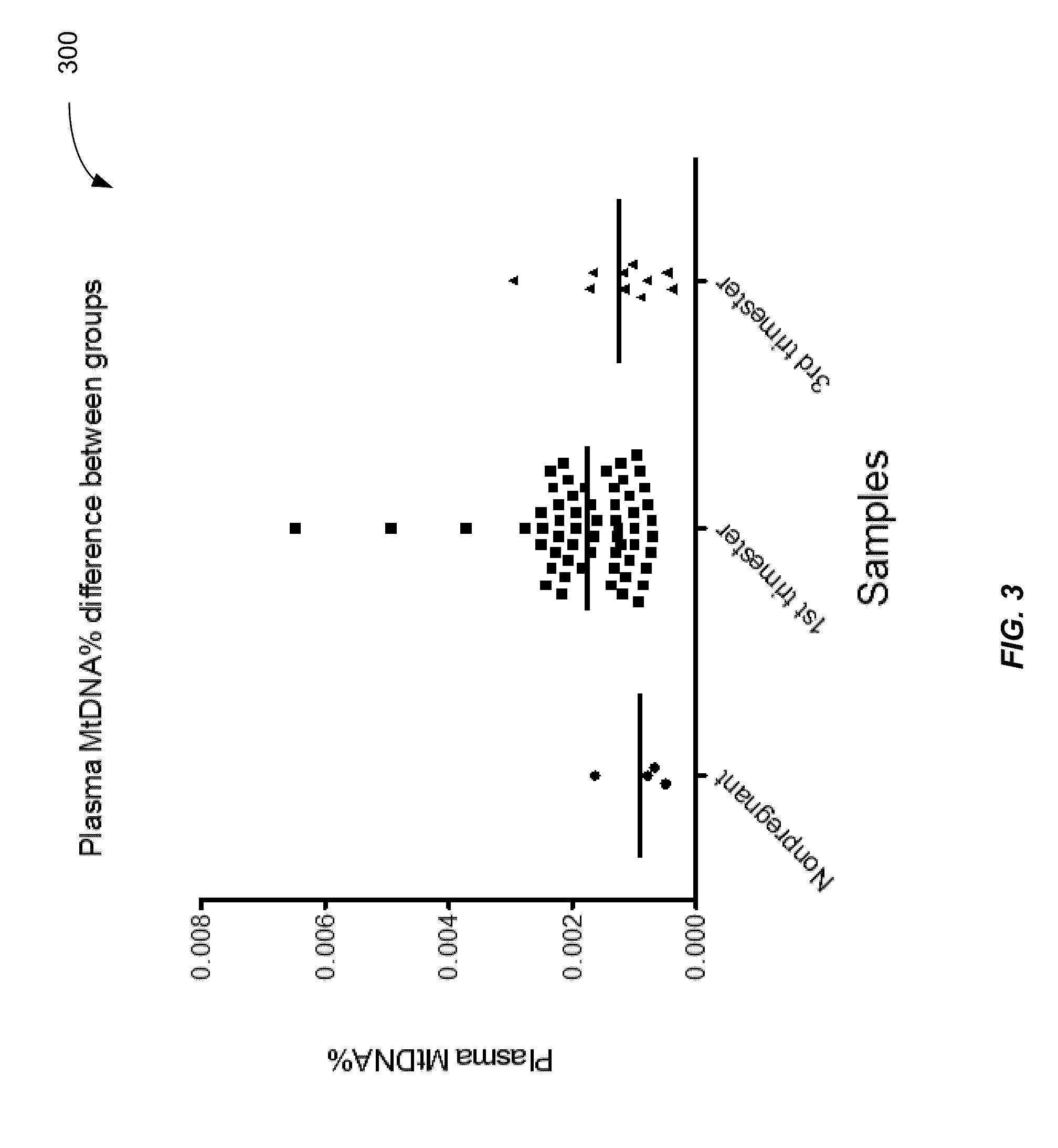
Applications of plasma mitochondrial DNA analysis
ActiveUS20160203260A1Reduce cost of measurementAccurately determineMicrobiological testing/measurementLibrary screeningAbnormal tissue growthTissues types
An amount of mitochondrial DNA molecules relative to an amount of nuclear DNA molecules is determined in a biological sample, and the relative amount is used for various purposes, e.g., screening, detection, prognostication or monitoring of various physiological and pathological conditions. As examples, an amount of mitochondrial DNA can be used to estimate a concentration of DNA of a tissue type, such as a fetal DNA concentration, tumor DNA concentration, or a concentration of DNA in the biological sample derived from a non-hematopoietic tissue source. Sequencing techniques can be used to determine a mitochondrial DNA concentration in a sample for an accurate detection of a level of cancer. A level of an auto-immune disease is also determined using a relative amount of mitochondrial DNA molecules compared nuclear DNA molecules.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Tumor DNA vaccines and virus vector vaccines with fibroblast activation protein alpha as target
ActiveCN105879060AIncreased growth inhibition rateGenetic material ingredientsNucleic acid vectorVector vaccineFibroblast activation protein, alpha
The invention relates to the field of tumor DNA vaccines and virus vector vaccines with fibroblast activation protein alpha as a target, in particular to recombinant DNA carrier CpVR-FAP, recombinant adenovirus Ad-FAP and recombinant poxvirus MVA-FAP vaccines, DNA vaccines, virus vector vaccines and optimal combination of immune ways of the virus vector vaccines or application of combination of the vaccines and cyclophosphamide in preparation of anti-tumor vaccines.
Owner:JILIN UNIV
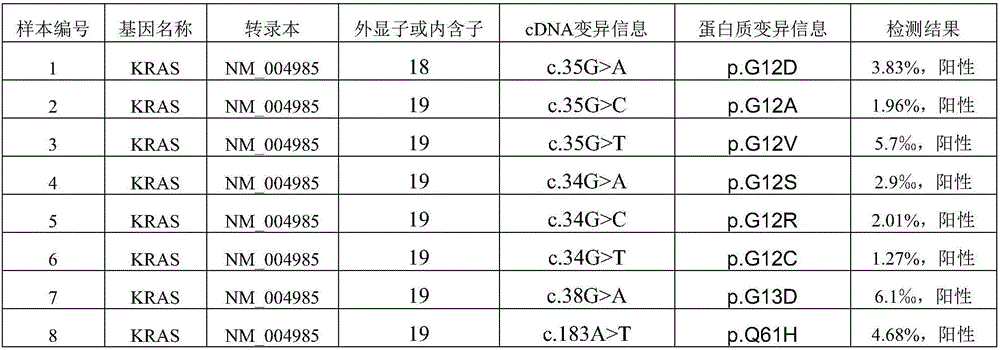
Capturing probe and kit for high-throughput sequencing detection of human circulating tumor DNA KRAS gene
ActiveCN106520963AGood hybridization effectImprove capture efficiencyMicrobiological testing/measurementDNA/RNA fragmentationKRASIntein
The invention relates to a capturing probe and kit for high-throughput sequencing detection of a human circulating tumor DNA KRAS gene. The human circulating tumor DNA KRAS gene capturing probe is a mixture of multiple kinds of probes which can capture different target regions on the human KRAS gene. In an optimized embodiment, the set of the probes can capture all coded exon regions and exon and intron boundary regions of the human circulating tumor DNA KRAS gene.
Owner:3D BIOMEDICINE SCI & TECH CO LTD
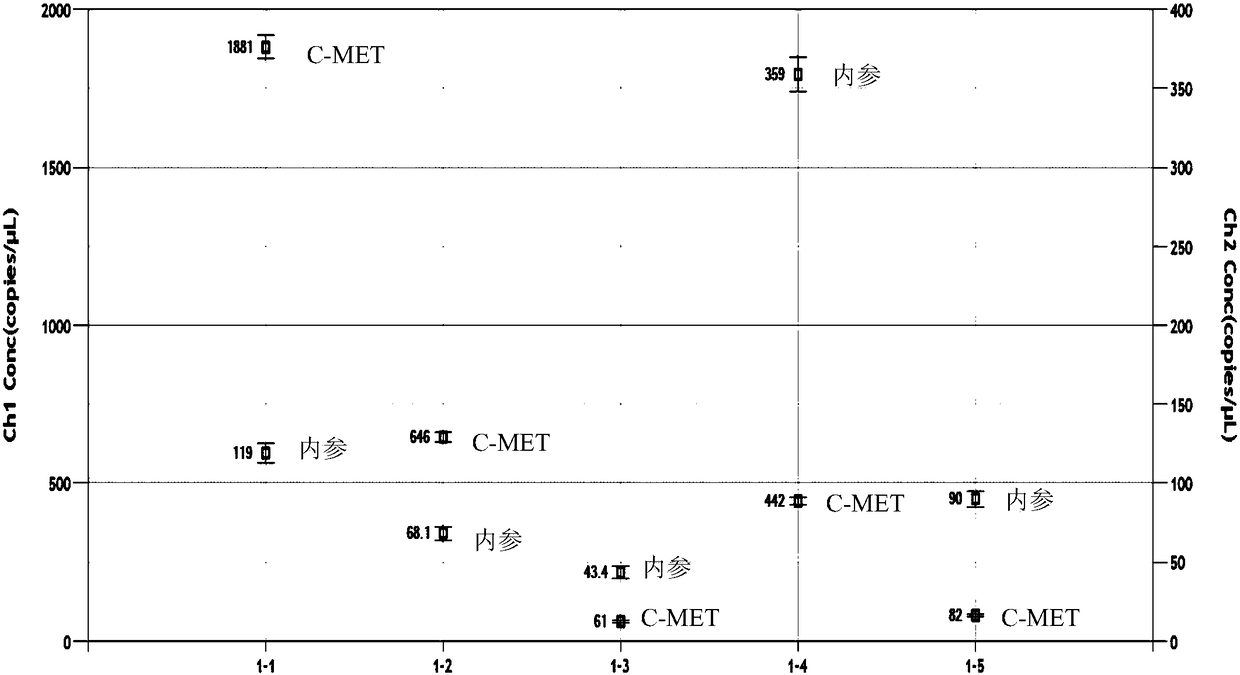
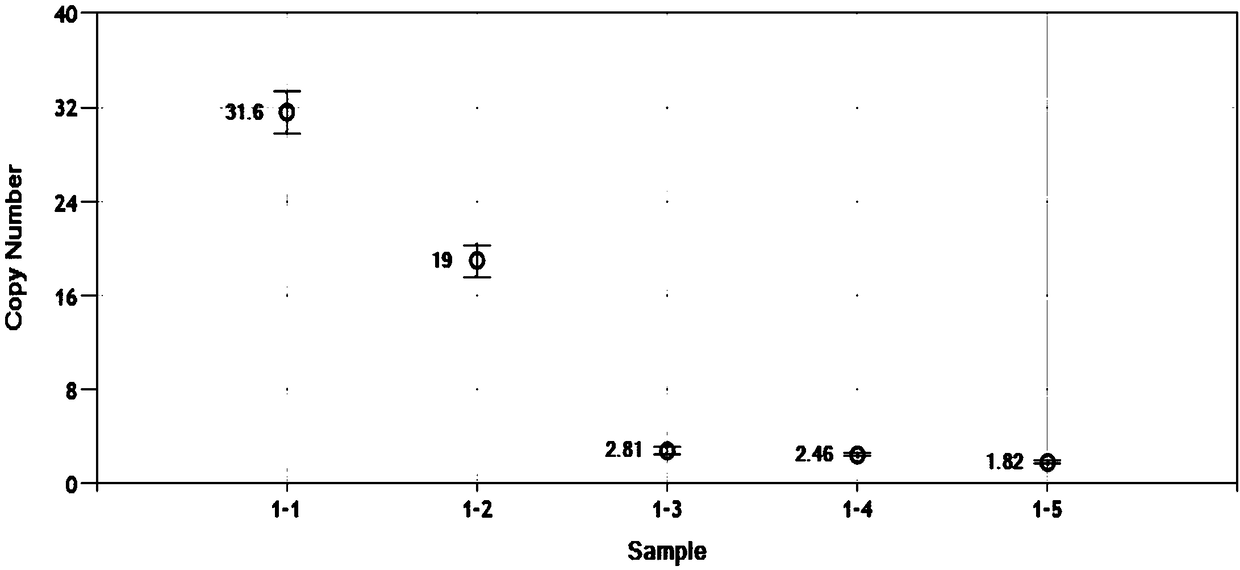
Primer and probe for detecting c-MET gene amplification through digital PCR technology and detection method thereof
InactiveCN108315431AStrong design specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationC-Met Gene AmplificationSmall fragment
Owner:PRIMBIO GENES BIOTECH WUHAN CO LTD
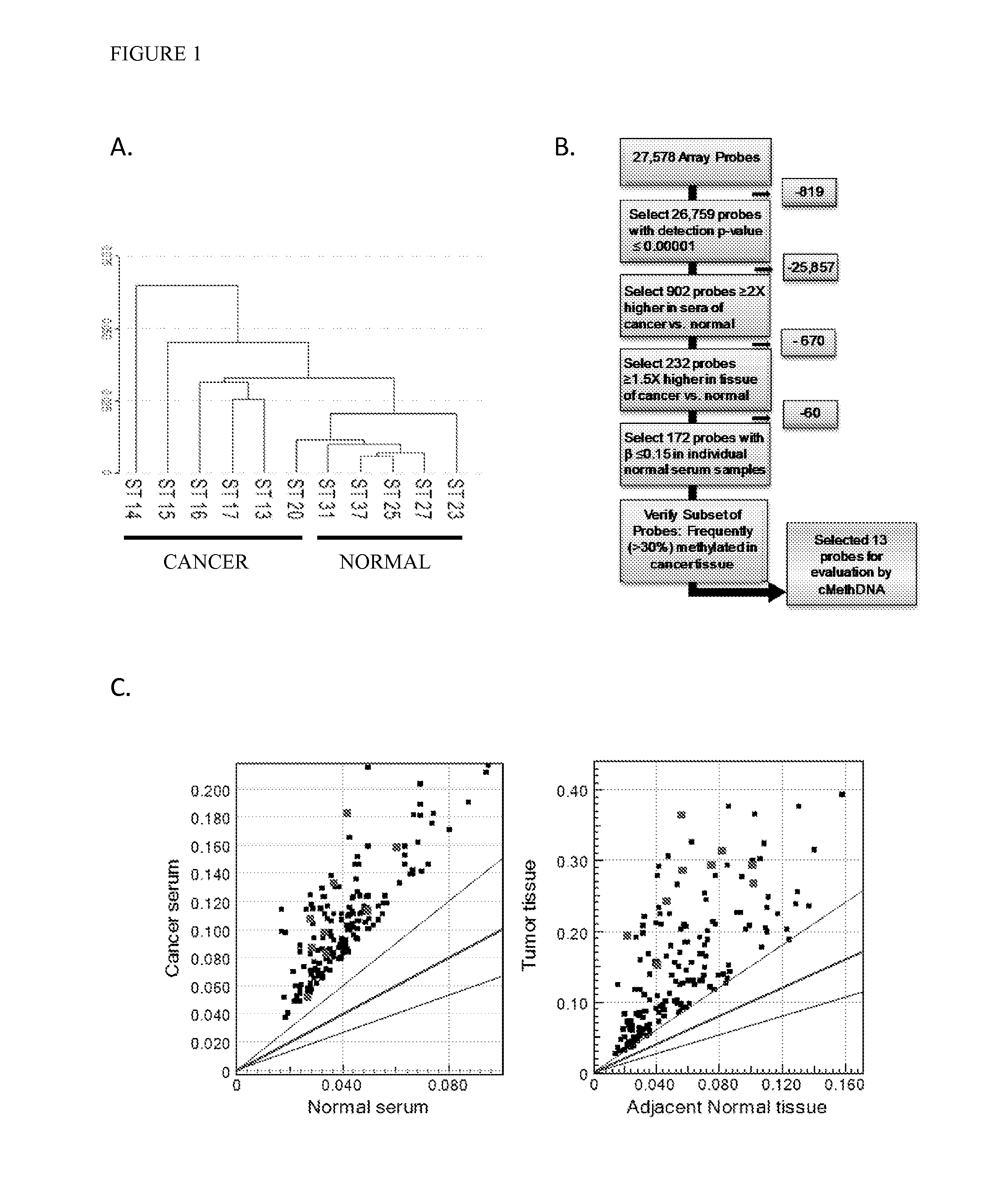
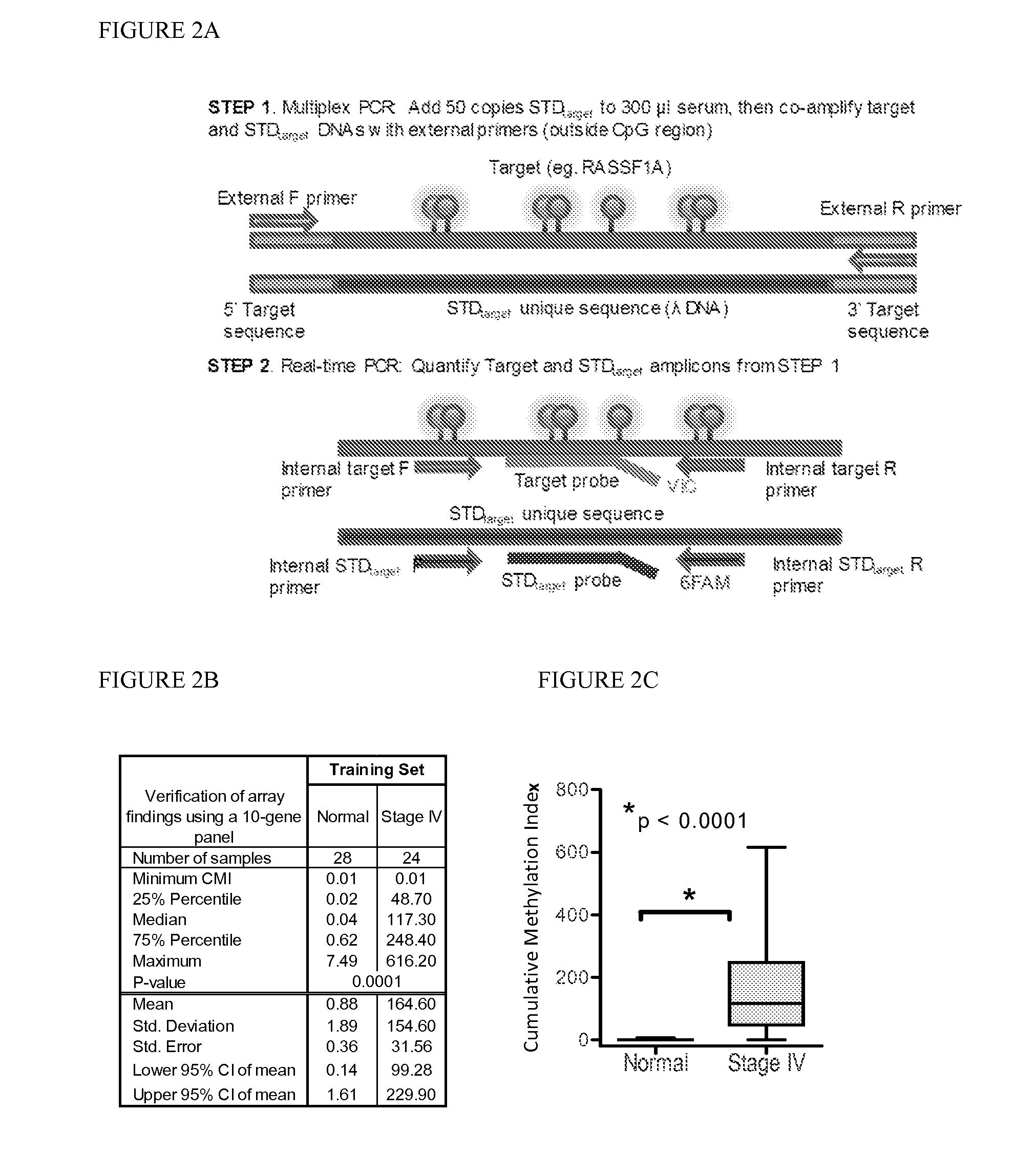
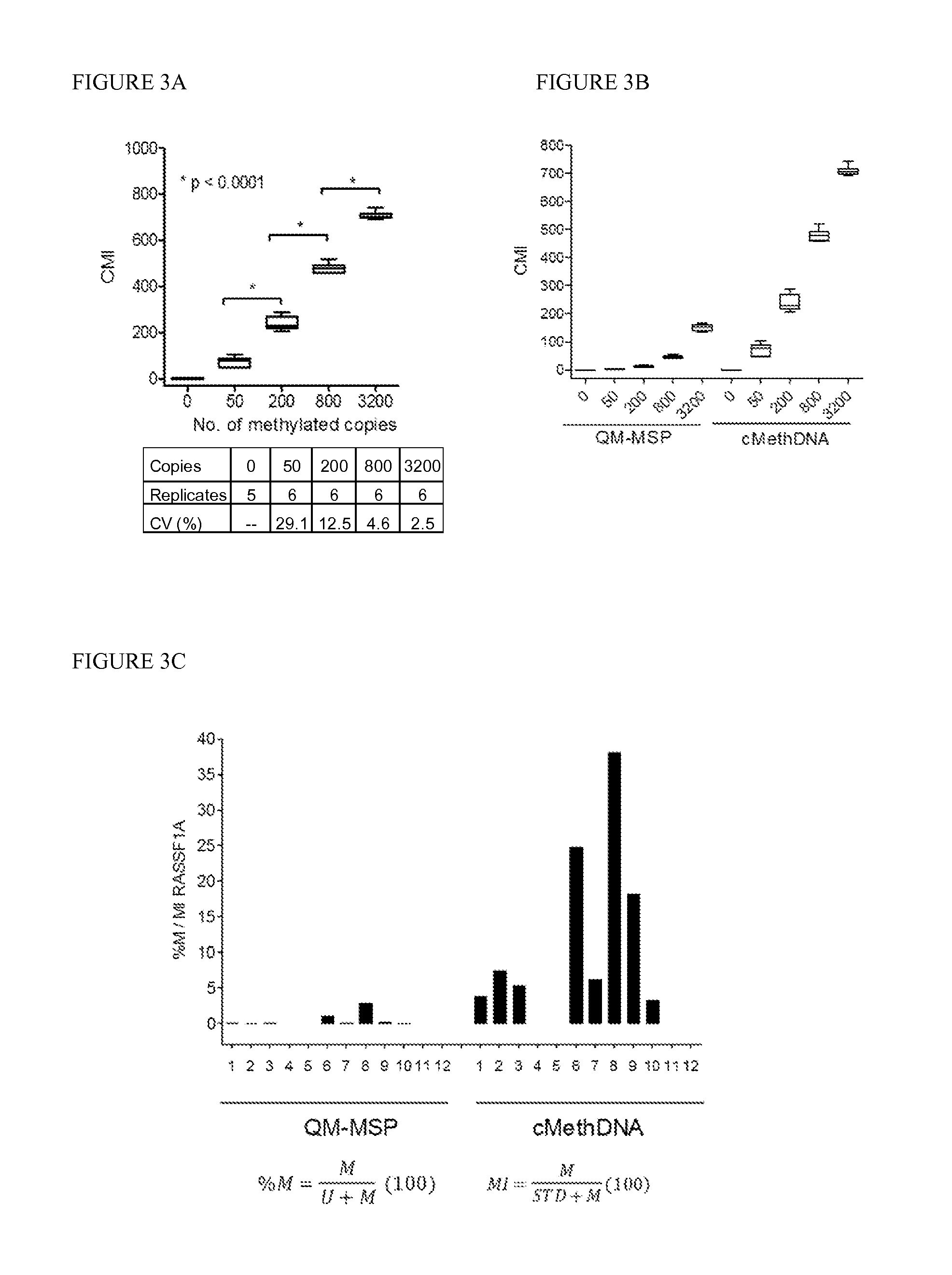
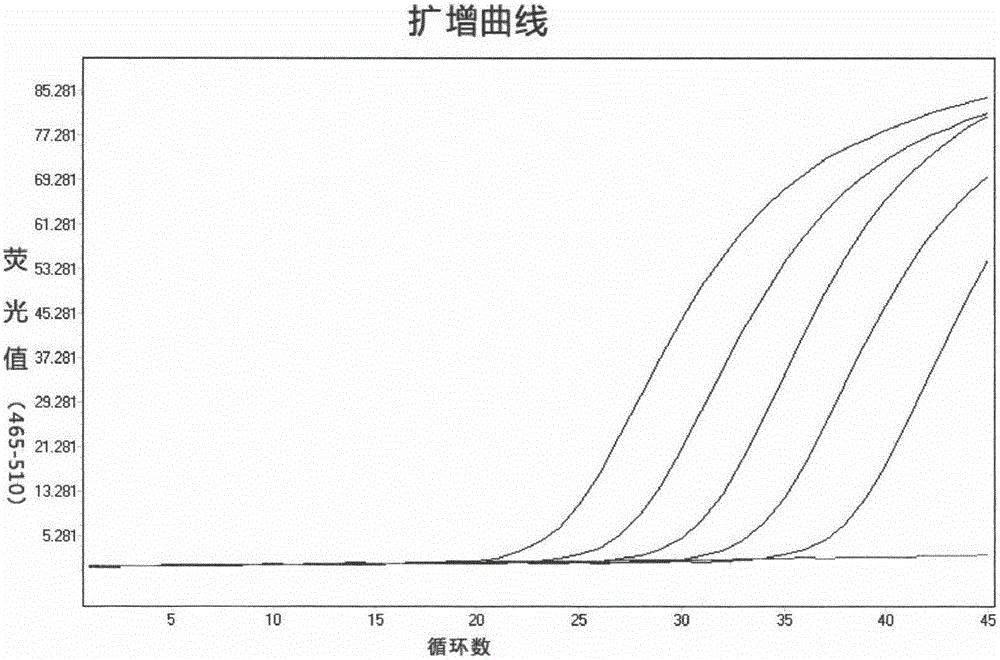
QUANTITATIVE MULTIPLEX METHYLATION SPECIFIC PCR METHOD- cMethDNA, REAGENTS, AND ITS USE
ActiveUS20150094222A1Evaluate responsePotential to evaluate response to treatmentSugar derivativesNucleotide librariesBlood plasmaPolynucleotide
The cMethDNA method of the present invention is a novel modification of the QM-MSP method (U.S. Pat. No. 8,062,849), specifically intended to quantitatively detect tumor DNA (or other circulating DNAs) in fluids such as serum or plasma at the lowest copy number yet reported. Unique compared to any other PCR-based assay, a small number of copies of a synthetic polynucleotide standard (STDgene) is added to an aliquot of patient serum. In a standard procedure, a cocktail of standards for a plurality of genes of interest (TARGETgene) is added to a sample of serum. Once total DNA is purified and processed, a PCR (multiplex step) is performed wherein the STDgene and the TARGETgene are co-amplified with the same external primer set. In the N second nested PCR step, amplicons present in a dilution of the first PCR reaction are subjected to real time PCR, and quantified for each gene in one well by two-color real-time PCR. Products are calculated by absolute quantitation with internal primer sets specific for the methylated TARGETgene and associated STDgene. Methods of making the STDgene standards and the use of the cMethDNA methods and kits containing the same are disclosed.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Tumor DNA (Deoxyribose Nucleic Acid) vaccine and virus vector vaccine taking mucoprotein 1 and surviving as targets
InactiveCN104013973AImprove immunityImprove tumor inhibition rateGenetic material ingredientsAntibody medical ingredients3-deoxyriboseVector vaccine
The invention relates to the field of tumor DNA (Deoxyribose Nucleic Acid) vaccines and virus vector vaccines, and in particular relates to recombinant DNA vectors VR-S8 and VR-MS, recombinant adenoviruses AD-S8 and Ad-MS and a recombinant poxvirus MVA-MS vaccine, as well as application of DNA vaccines and optimized combination of immunization ways between virus vector vaccines to preparation of antitumor vaccines.
Owner:CHANGCHUN BCHT BIOTECH
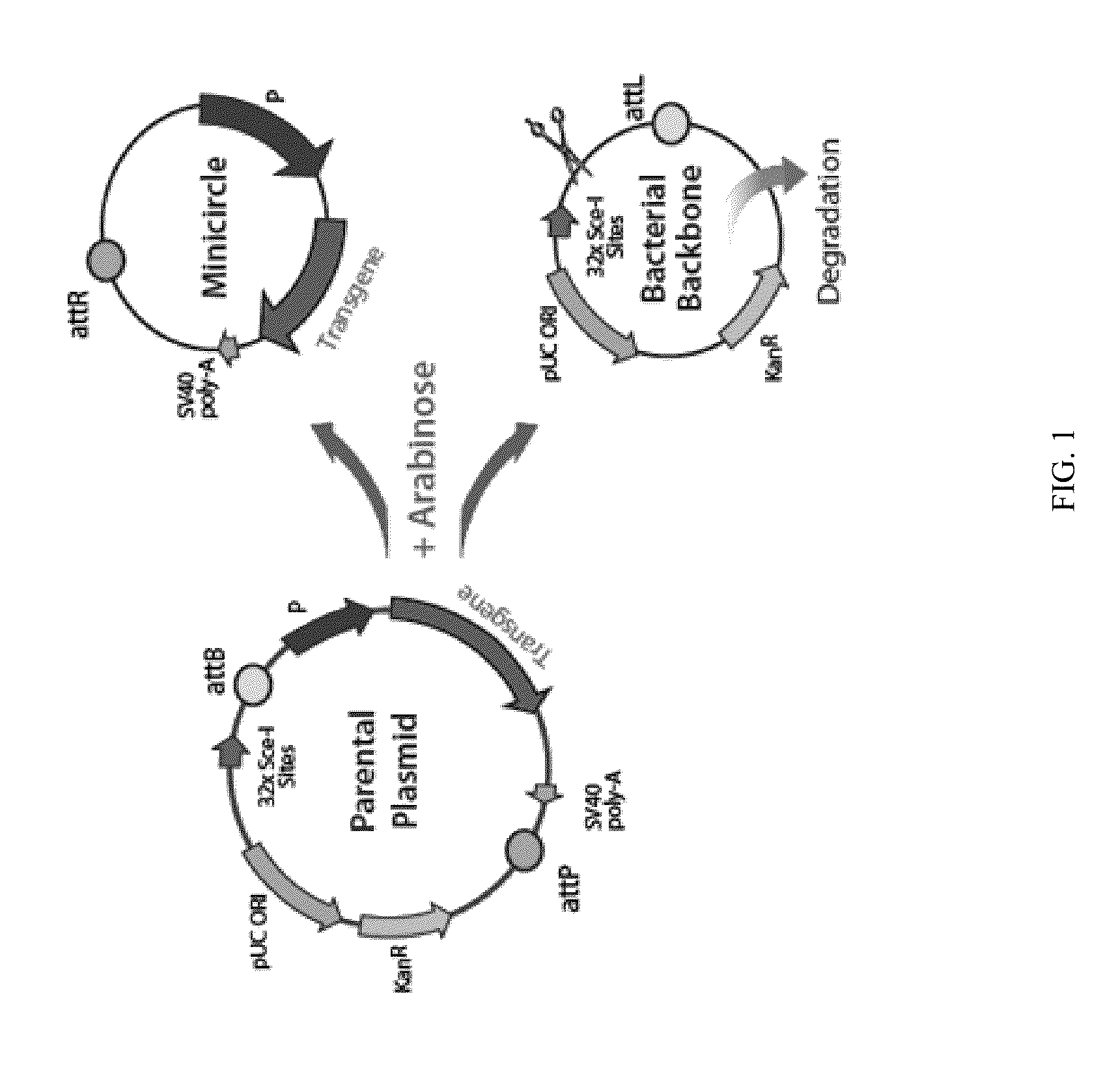
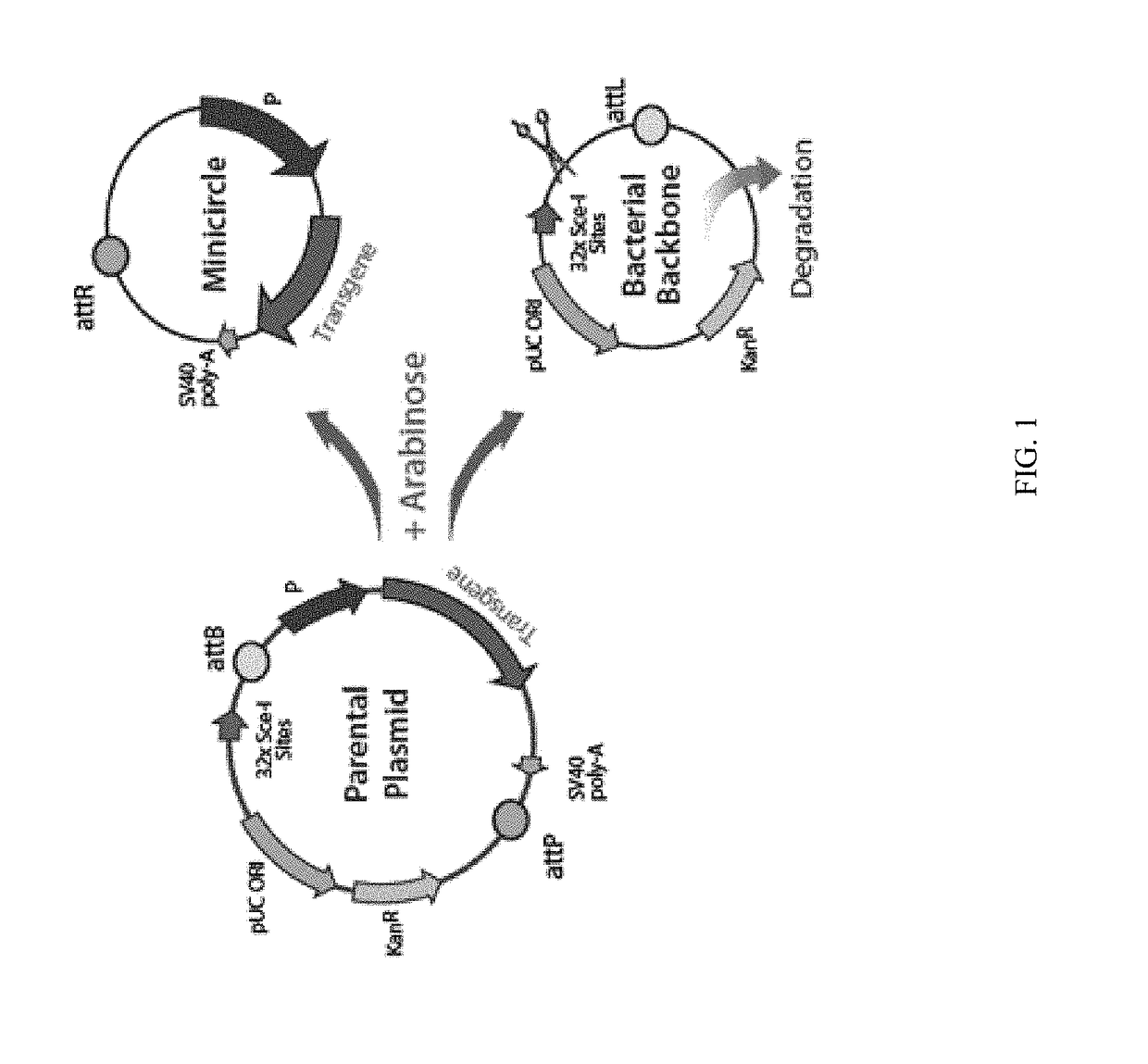
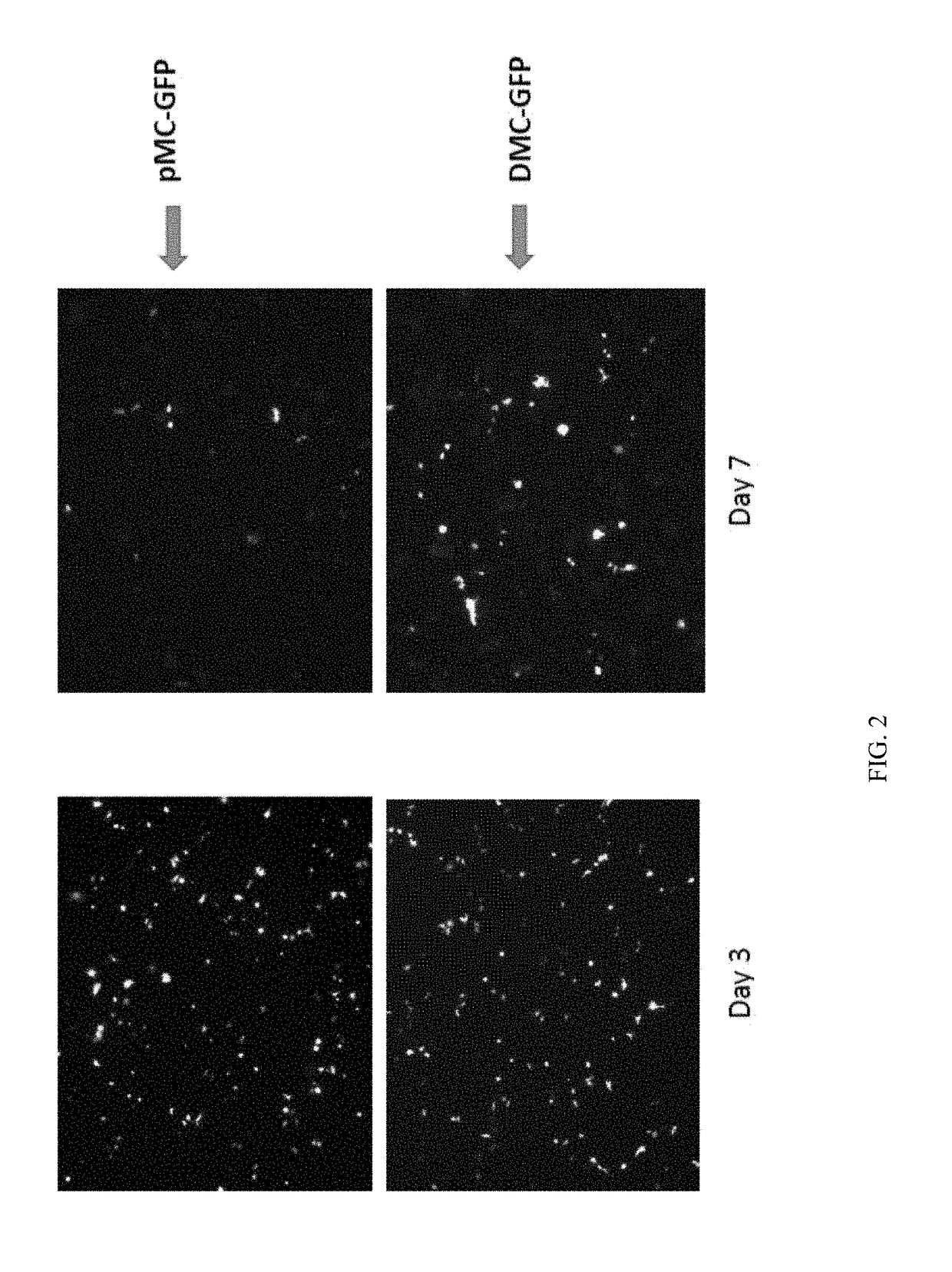
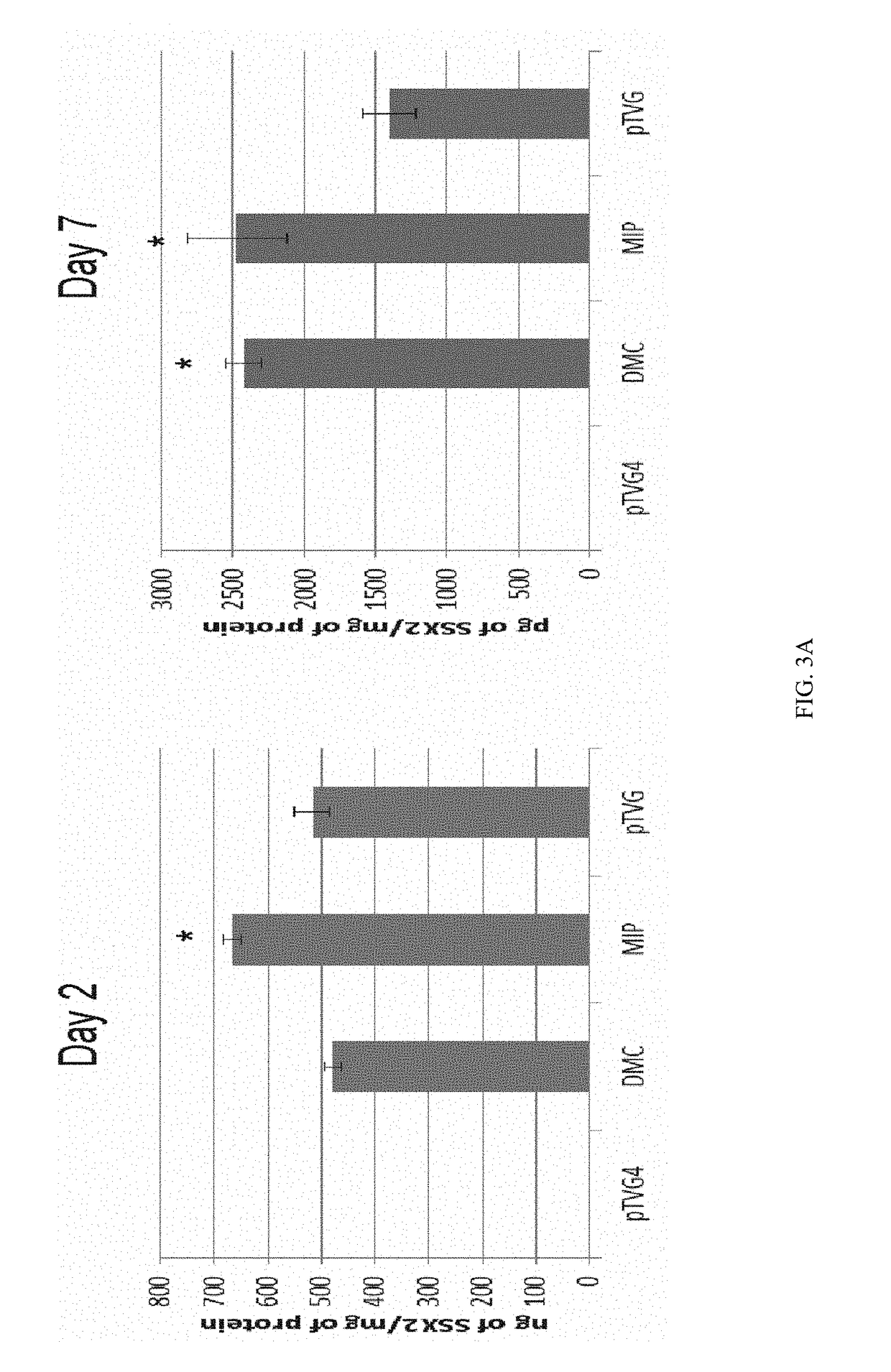
Mini-intronic plasmid DNA vaccines in combination with lag3 blockade
ActiveUS20160166686A1Reduce the number of cellsAntibody ingredientsNucleic acid vectorInteinPlasmid dna
Owner:WISCONSIN ALUMNI RES FOUND
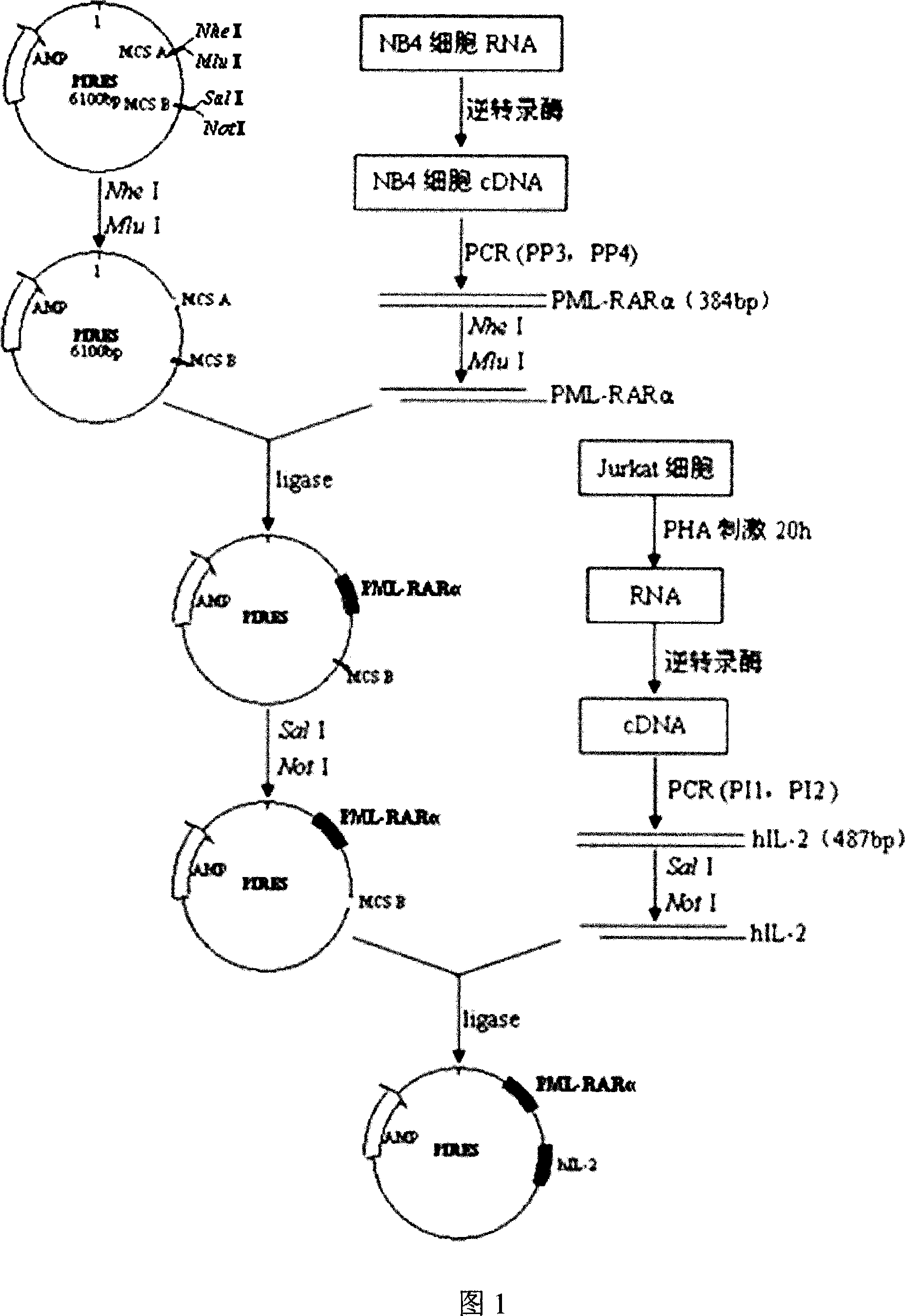
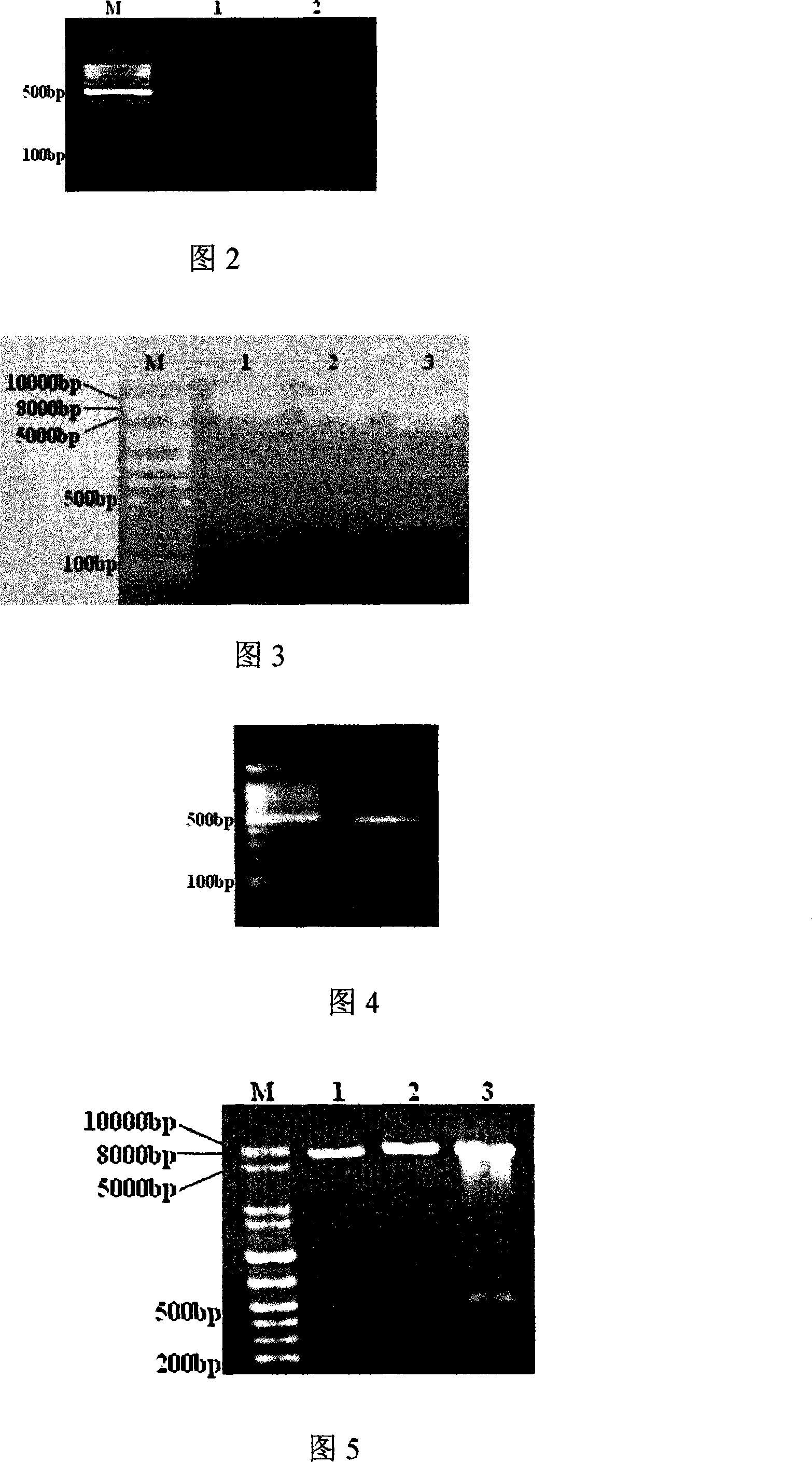
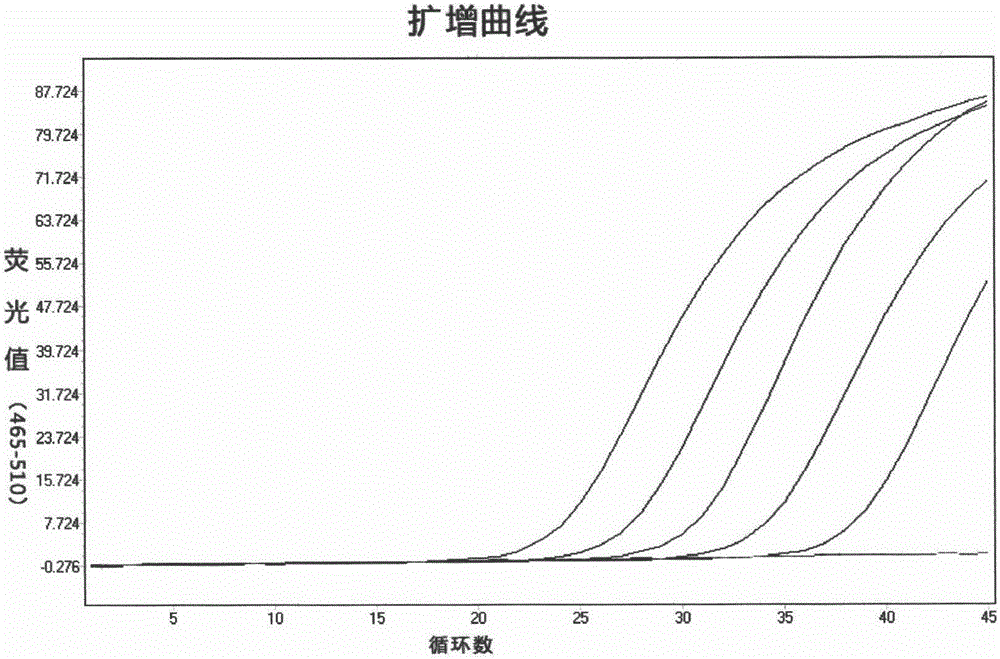
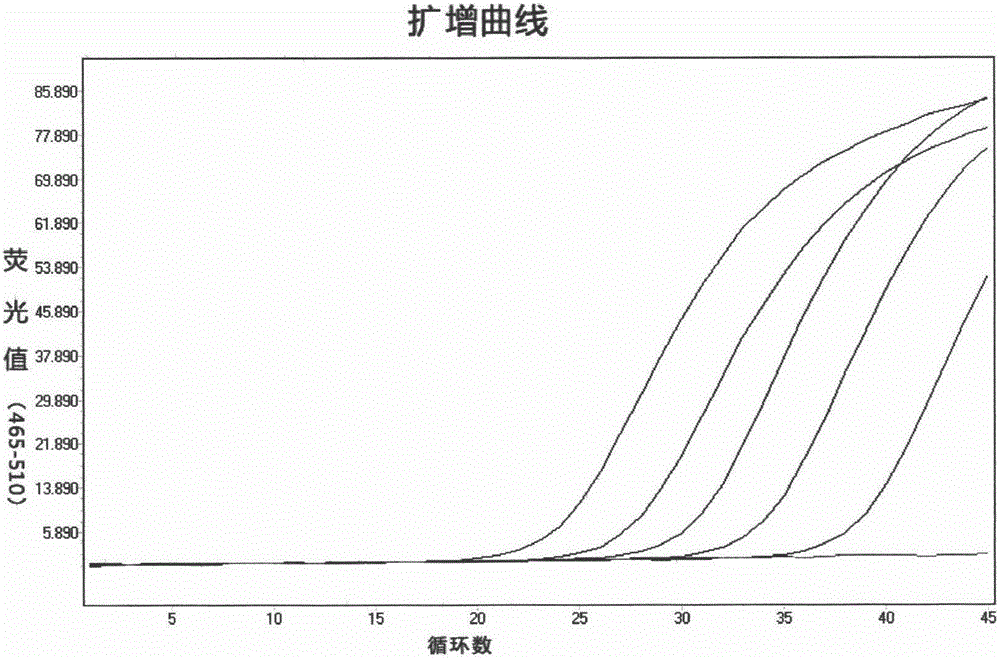
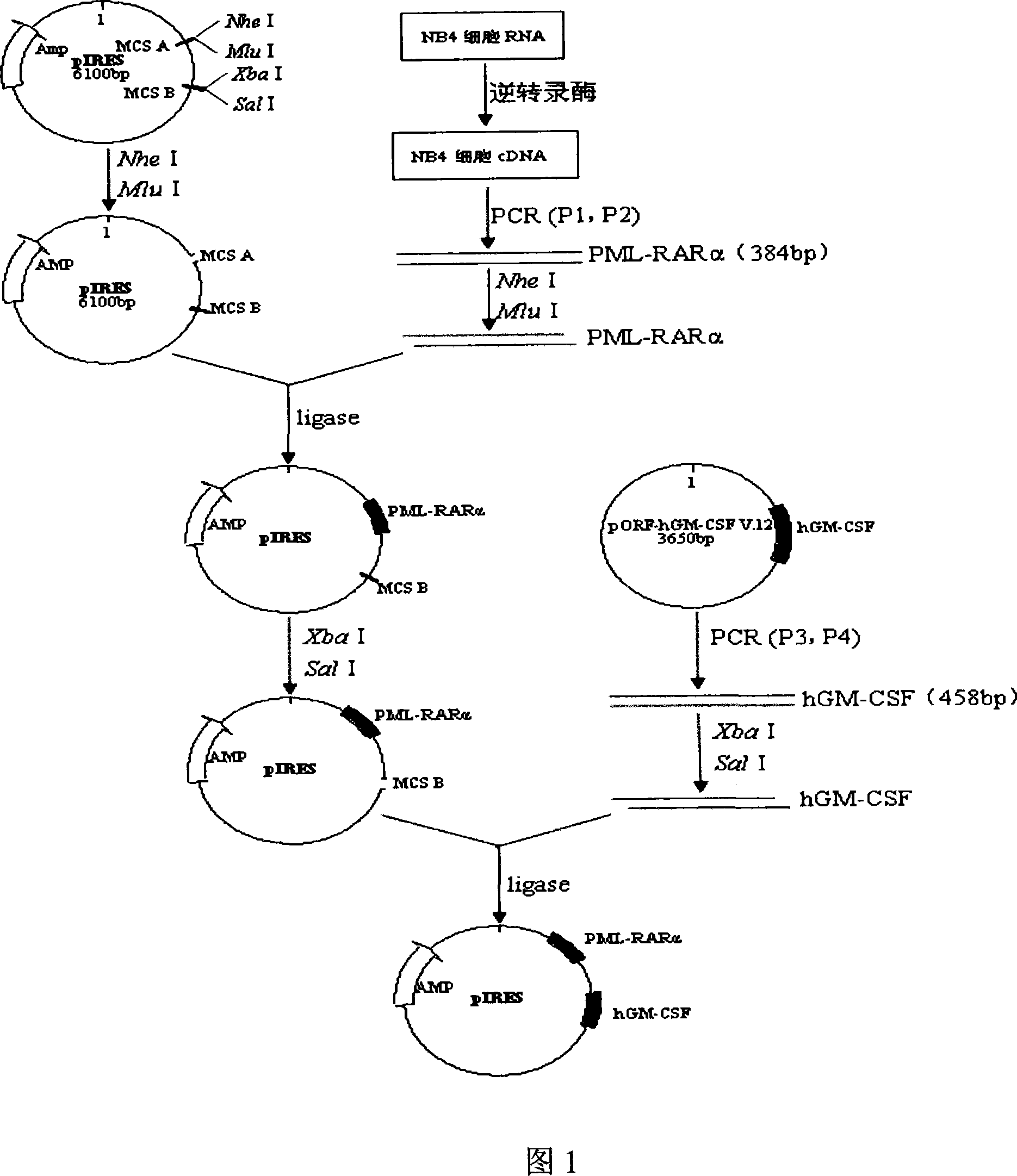
Acute progranulocyte leukemia DNA vaccine PML-RAR alpha 384-hIL-2 and preparing method and application thereof
InactiveCN101224307AEradication of minimal residual diseaseTo achieve the purpose of curing APLGenetic material ingredientsAntineoplastic agentsMRD NegativeImmune effects
The invention discloses acute promyelocytic leukemia DNA vaccine PML-RAR Alpha 384-hIL-2 which provides the fusion gene pIRES-PML-RAR Alpha-hIL-2 and also the medicine for the application of the DNA vaccine to prepare acute promyelocytic leukemia. The invention constructs DNA vaccine by using the PML-RAR Alpha fusion gene to eradicate the MRD of the APL provides PML-RAR Alpha-hIL-2 double-gene DNA vaccine which can induce and produce the PML-RAR Alpha-hIL-2 with specific anti APL immune effect and provides important data and information for the research of DNA vaccine that cures the APL. The method of the invention can also be referred by other research institutes on curing tumor DNA vaccine; the DNA vaccine of the invention can clear the minimal residual diseases of the bodies of APL patients, so as to finally achieve the aim of curing the APL.
Owner:JINAN UNIVERSITY
Personalized, economical and practical tumor therapeutic effect evaluation and relapse monitoring method
InactiveCN105018634ASolve the detection sensitivity problemHigh sensitivityMicrobiological testing/measurementPersonalizationFluorescence
The invention provides a non-invasive tumor therapeutic effect evaluation and relapse monitoring method which is economical, practical, high in sensitivity and effective for each individual. The method includes the steps of one-time high-flux sequencing screening and real-time fluorescence PCR daily non-invasive monitoring. Firstly, tumor DNA is extracted from tumor tissue or blood or marrow of individuals, sequencing is conducted through a high-flux sequencing instrument, proper detection sites are screened according to a screening standard provided by the method, a real-time fluorescence PCR method is established, and the relapse conditions of tumors are monitored and therapeutic effect evaluation is conducted through existence of the detection sites in the blood. The bodies of the patients are searched for existing mutational sites through a high-flux sequencing method, and it can be guaranteed that the method is effective for each individual. The detection sites screened according to the special screening standard provided by the method can be detected in the background of a large amount of normal DNA, and sensitivity is high. The real-time fluorescence PCR method serves as a means for daily monitoring tumor mutant genes in the blood or urine, and the method is economical, practical and free of trauma.
Owner:SHANGHAI ACEBIOX BIOTECHNOLOGY CO LTD
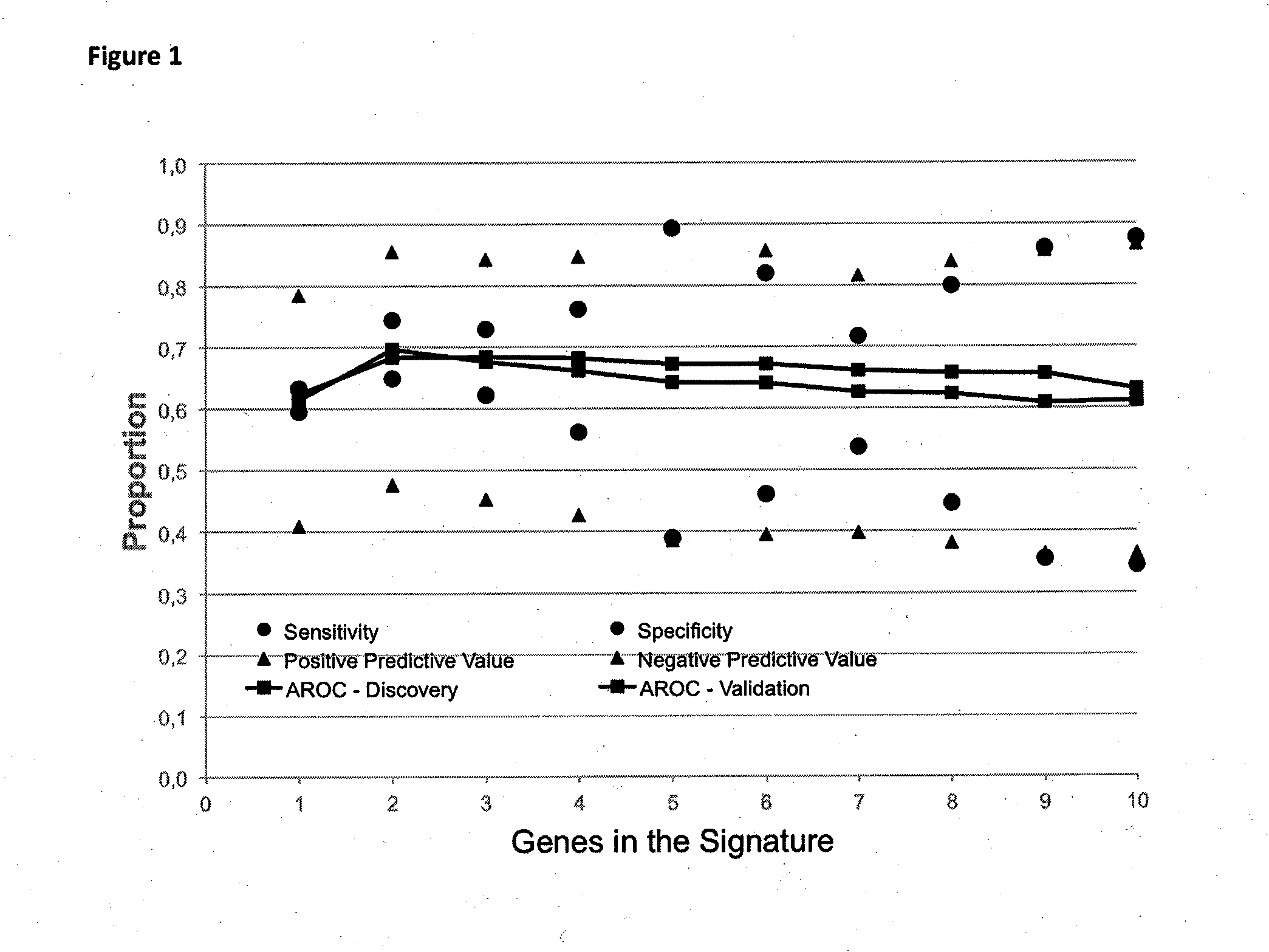
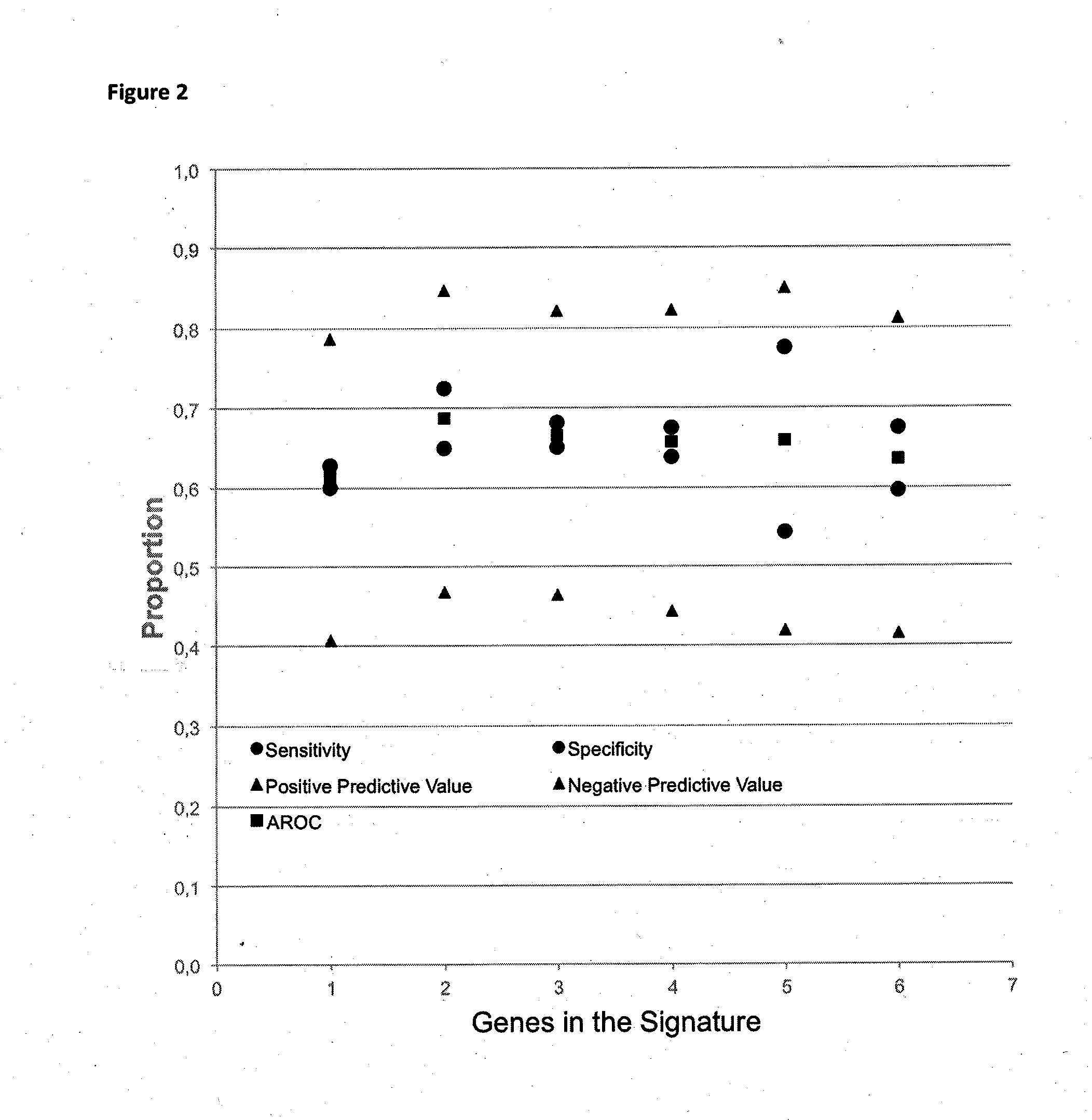
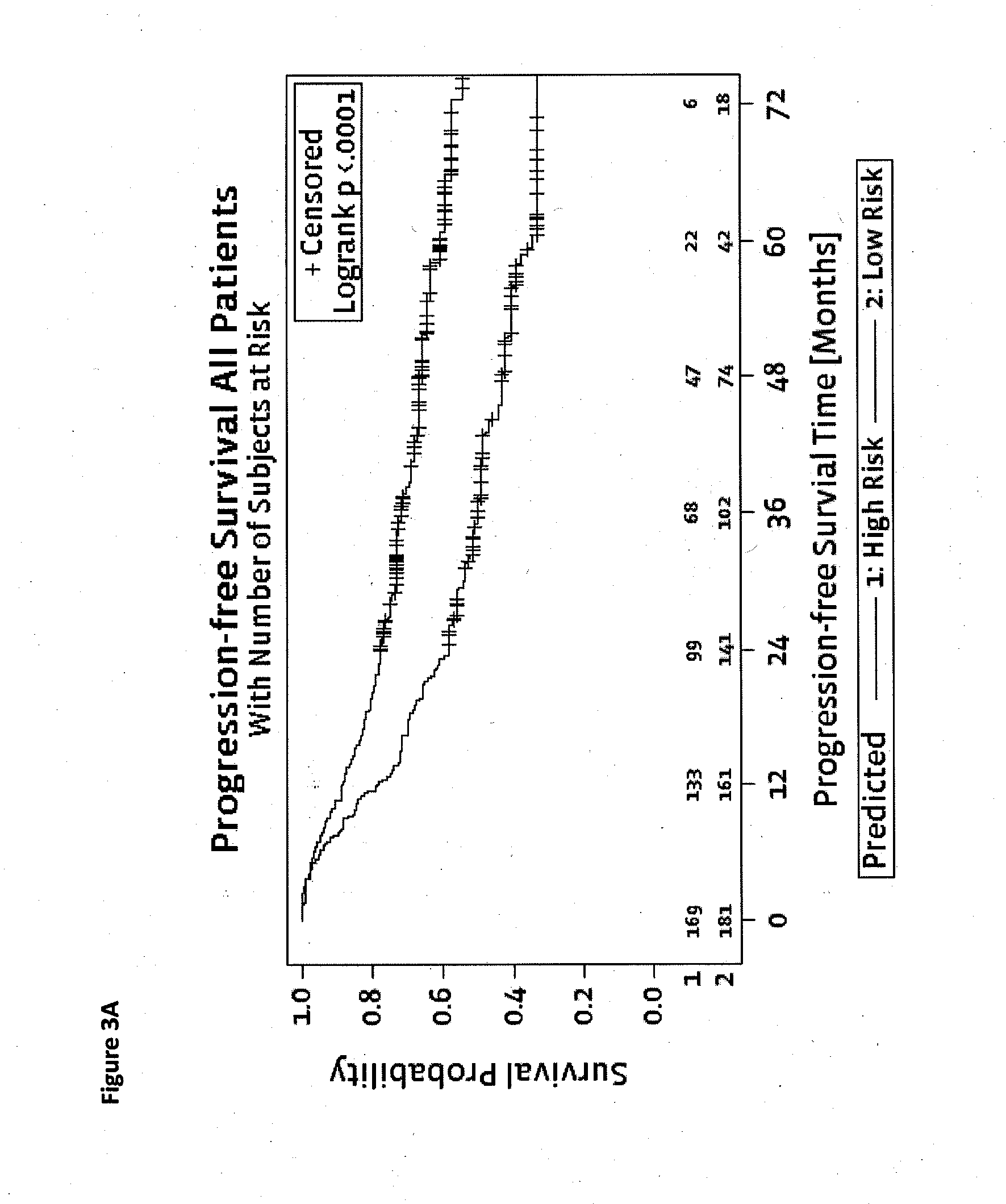
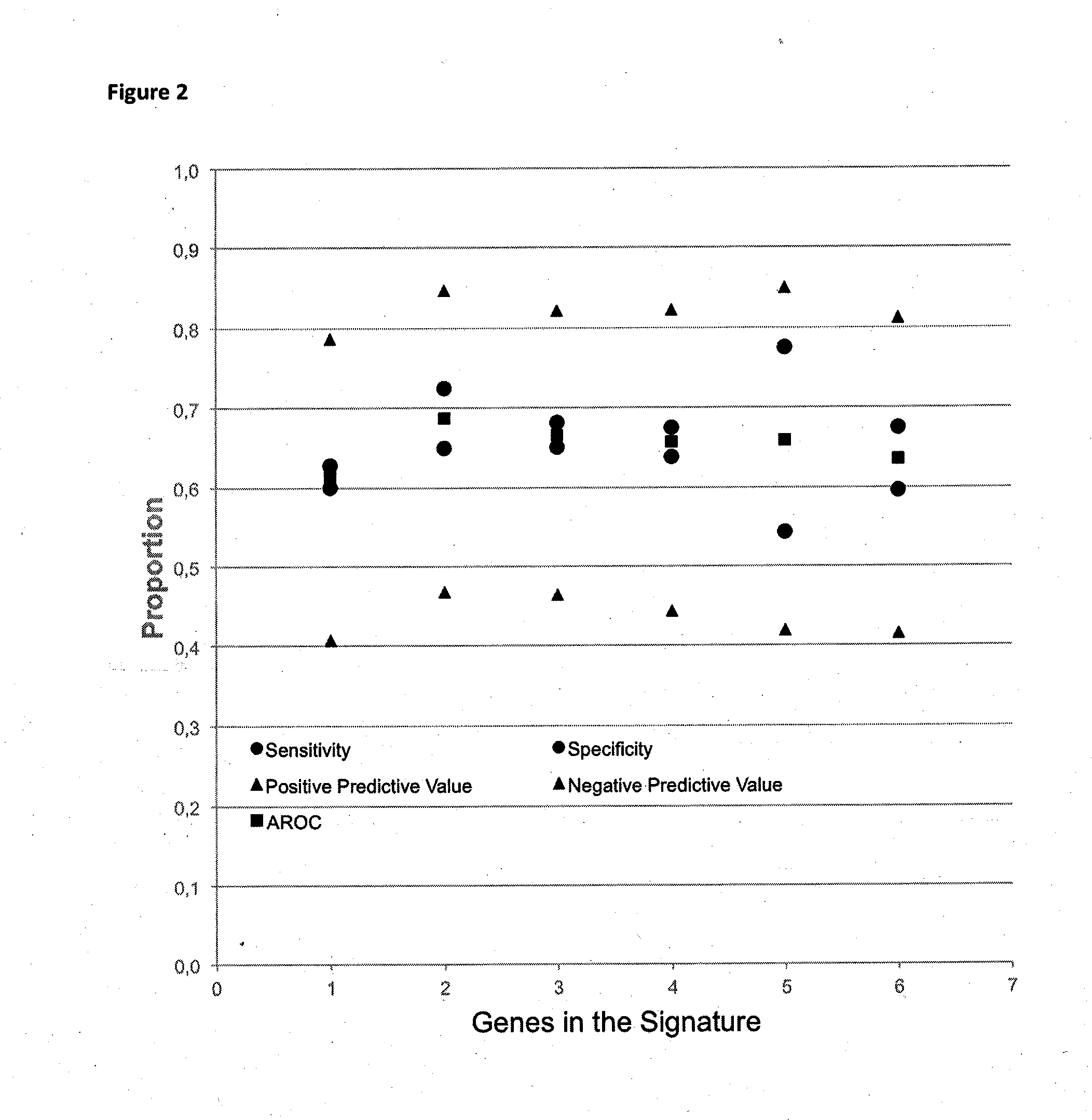
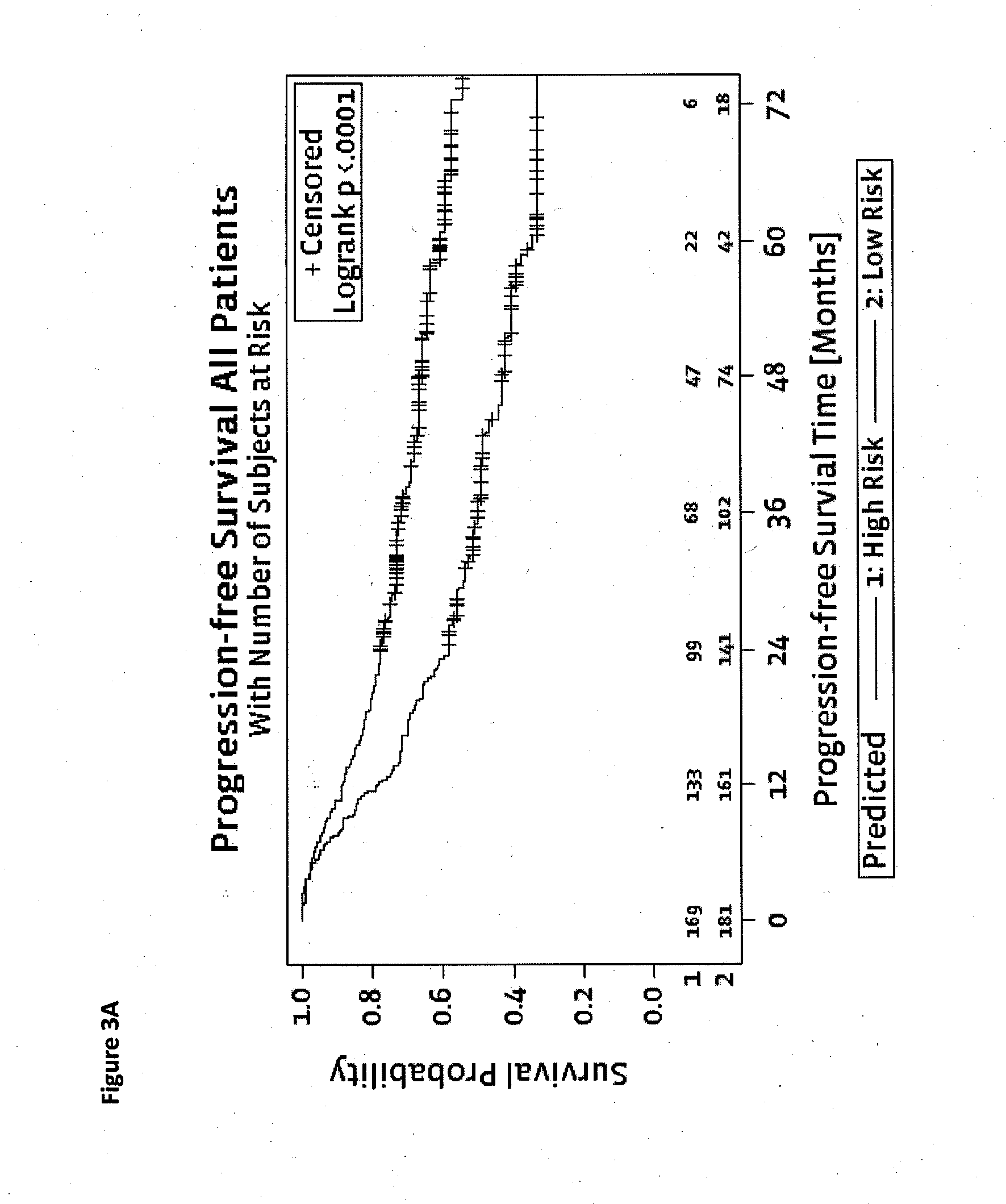
Method for predicting a manifestation of an outcome measure of a cancer patient
InactiveUS20160153032A9Nucleotide librariesMicrobiological testing/measurementOutcome measuresTissue sample
The invention pertains to a method for predicting a manifestation of an outcome measure of a cancer patient based on a tumor DNA containing tissue sample from the cancer patient, comprising, firstly, determining an existence of a sequence variation within segments of at least two genes of the tumor DNA as Present, if at least one significant sequence variation can be determined, or as Absent, if no significant sequence variation can be determined, wherein the at least two genes of the tumor DNA are associated with the outcome measure of the patient; secondly, combining the existence of sequence variations of the at least two genes using a logical operation (prediction function), and thirdly, predicting based on the results of the logical operation the manifestation of an outcome measure of the patient.
Owner:SIGNATURE DIAGNOSTICS
Head and neck squamous cell carcinoma assays
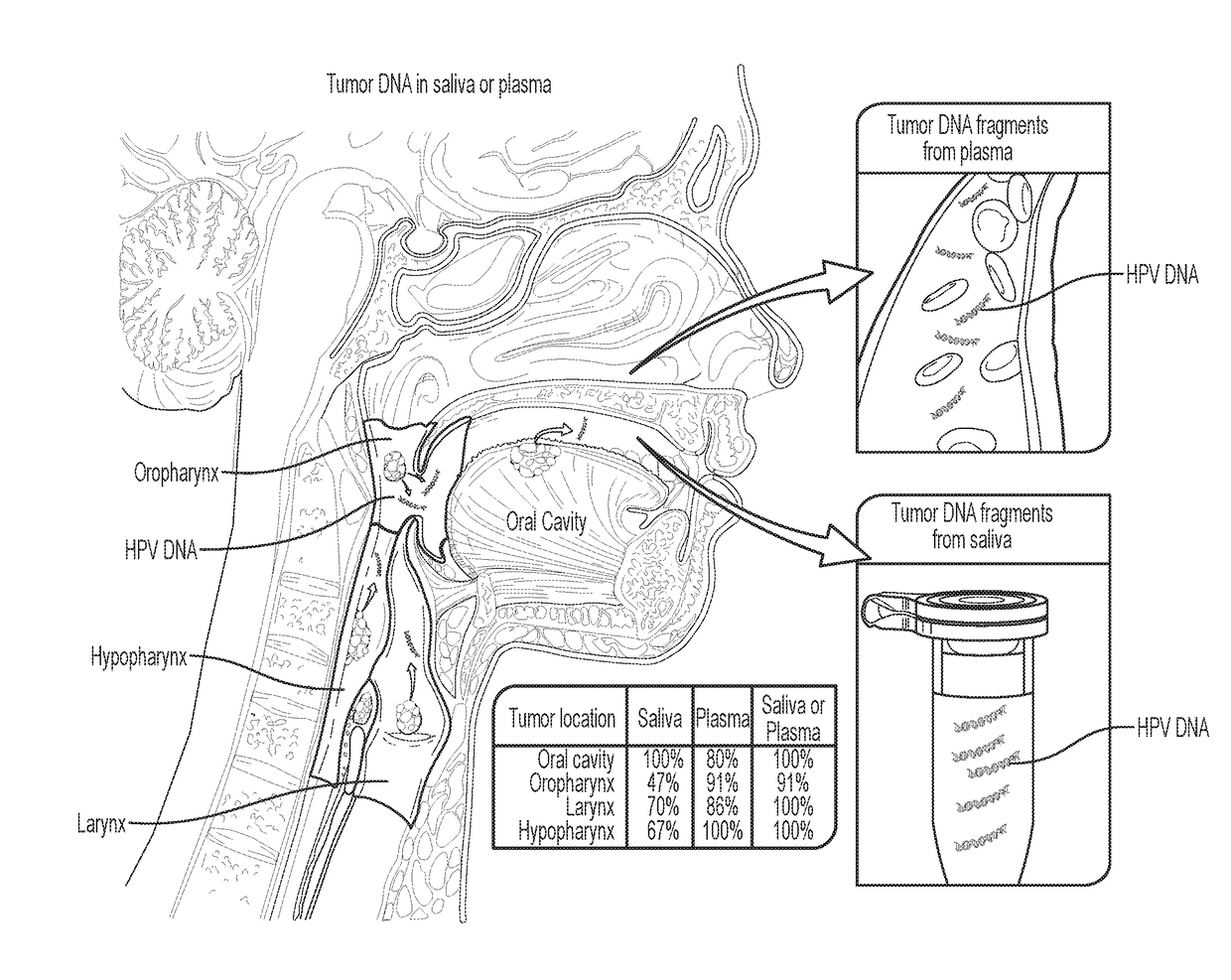
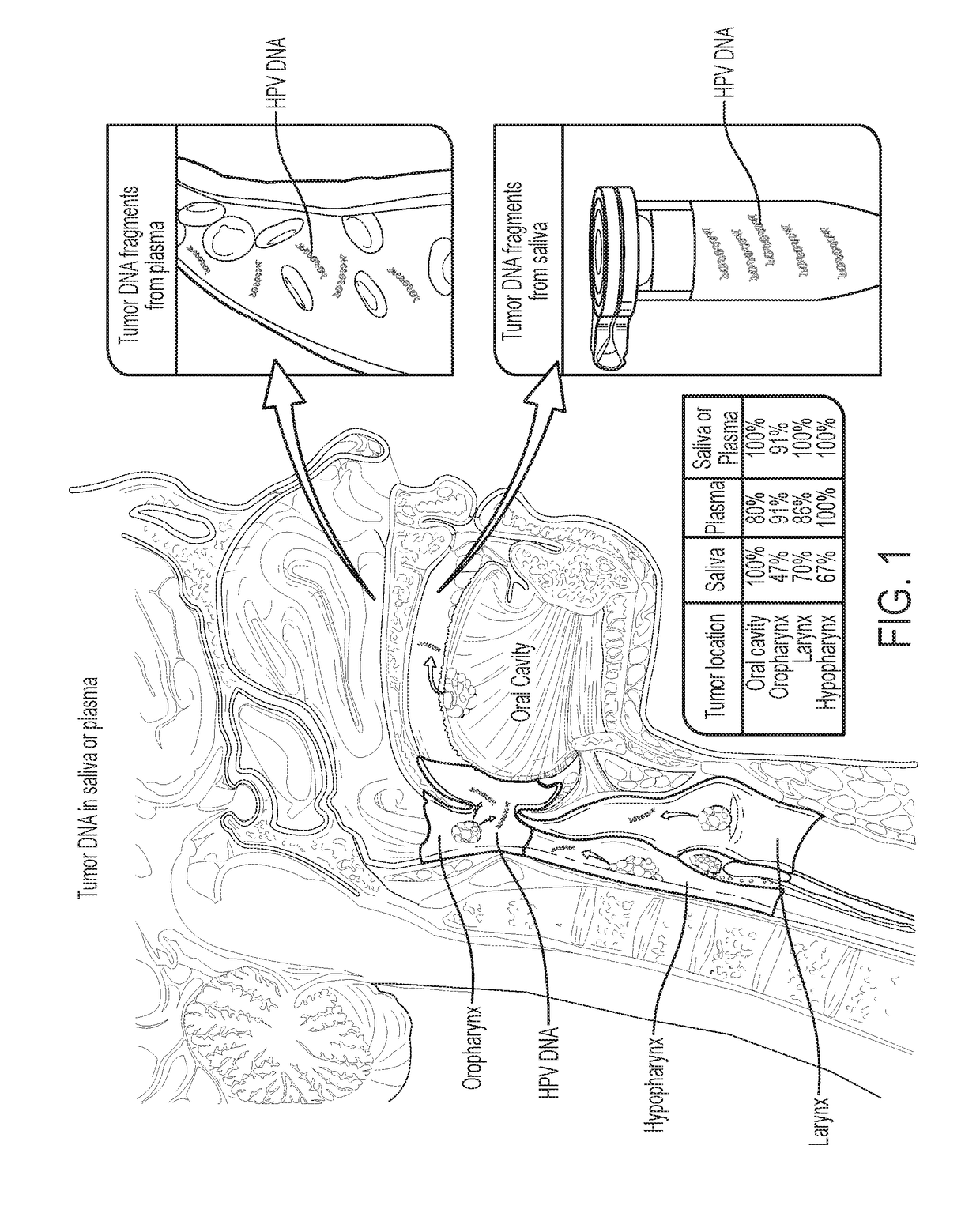
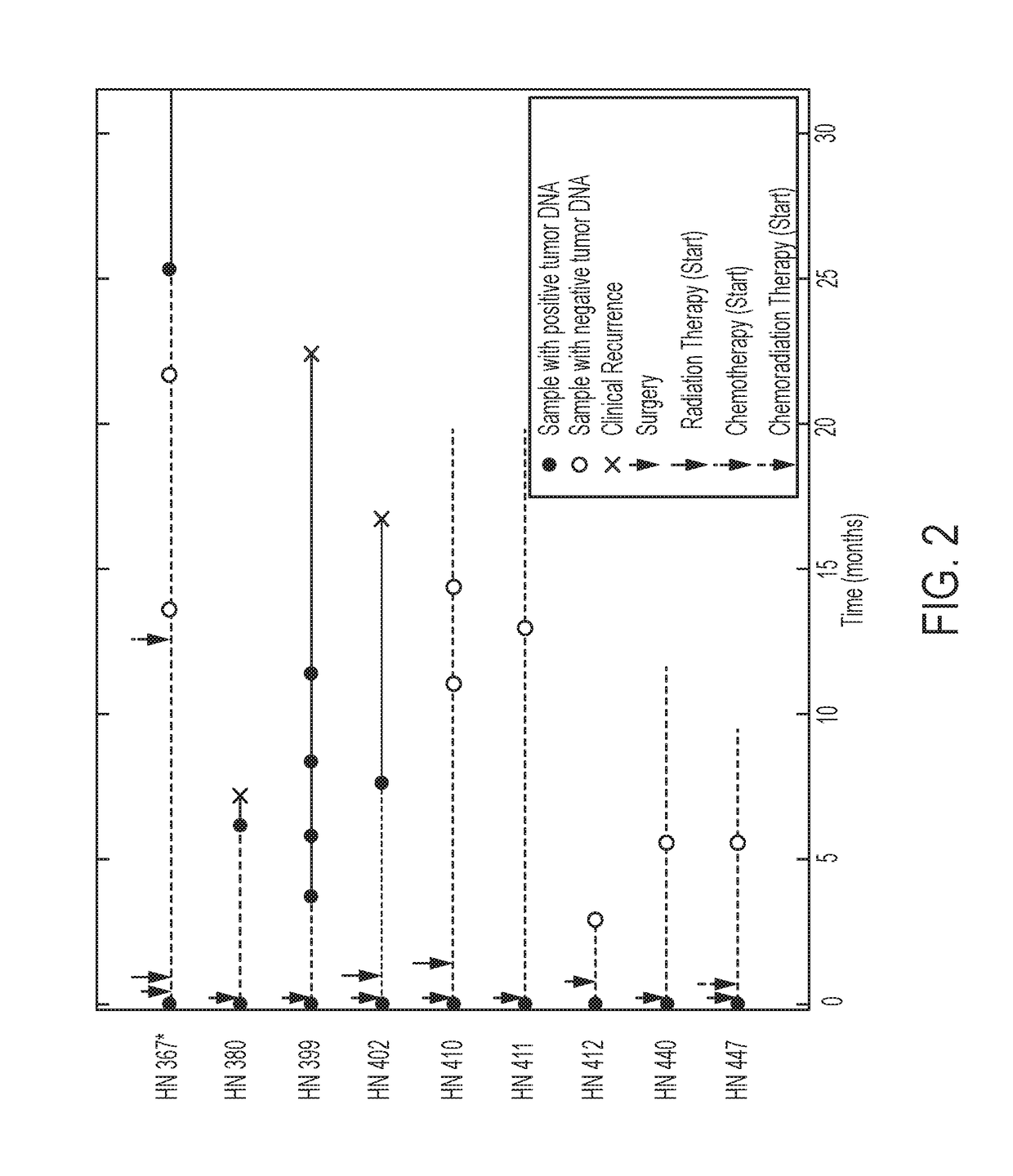
We queried DNA from saliva or plasma of 93 HNSCC patients, searching for somatic mutations or human papillomavirus genes, collectively referred to as tumor DNA. When both plasma and saliva were tested, tumor DNA was detected in 96% (95% CI, 84% to 99%) of 47 patients. The fractions of patients with detectable tumor DNA in early- and late-stage disease were 100% (n=10) and 95% (n=37), respectively. Saliva is preferentially enriched for tumor DNA from the oral cavity, whereas plasma is preferentially enriched for tumor DNA from the other sites. Tumor DNA in the saliva and plasma is a valuable biomarker for detection of HNSCC.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for predicting a manifestation of an outcome measure of a cancer patient
InactiveUS20140342925A1Nucleotide librariesMicrobiological testing/measurementOutcome measuresLogical operations
The invention pertains to a method for predicting a manifestation of an outcome measure of a cancer patient based on a tumor DNA containing tissue sample from the cancer patient, comprising, firstly, determining an existence of a sequence variation within segments of at least two genes of the tumor DNA as Present, if at least one significant sequence variation can be determined, or as Absent, if no significant sequence variation can be determined, wherein the at least two genes of the tumor DNA are associated with the outcome measure of the patient; secondly, combining the existence of sequence variations of the at least two genes using a logical operation (prediction function), and thirdly, predicting based on the results of the logical operation the manifestation of an outcome measure of the patient.
Owner:SIGNATURE DIAGNOSTICS
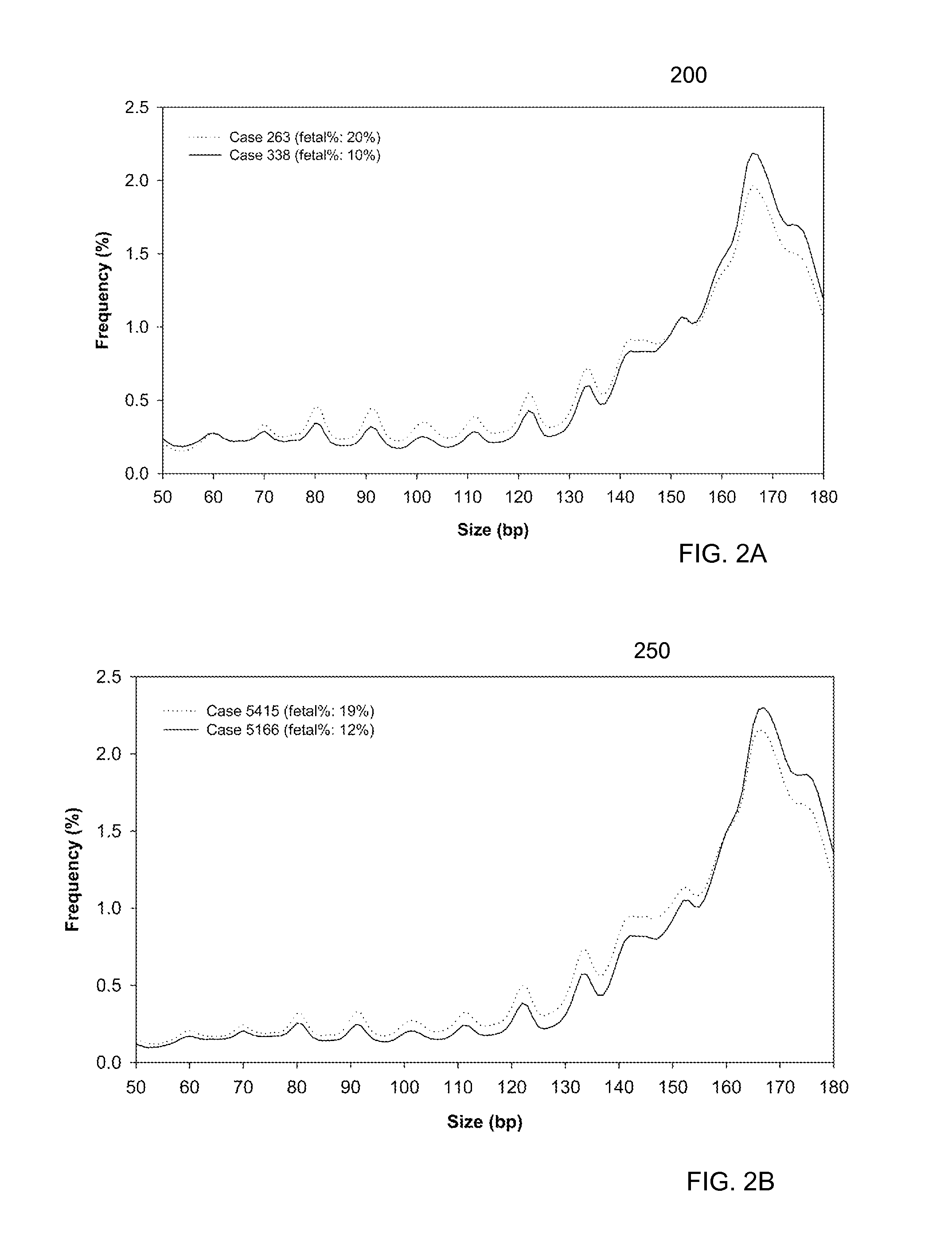
Size-based analysis of tumor DNA
ActiveUS20170132363A1Health-index calculationMicrobiological testing/measurementAbnormal tissue growthBiological body
A classification of a level of cancer in an organism is determined by analyzing a biological sample of the organism. The biological sample comprises clinically-relevant DNA and other DNA. At least some of the DNA is cell-free in the biological sample. An amount of a first set of DNA fragments from the biological sample corresponding to each of a plurality of sizes is measured. A first value of a first parameter is calculated based on the amounts of DNA fragments at the plurality of sizes. The first value is compared to a reference value. A classification of a level of cancer in the organism is determined based on the comparison.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Acute promyelocytic leukemia DNA vaccine PML-RAR alpha-hGM-CSF and preparation and application thereof
InactiveCN101214381AEradication of minimal residual diseaseTo achieve the purpose of curing APLGenetic material ingredientsAntineoplastic agentsMRD NegativeFhit gene
The invention discloses an acute promyelocytic leukemia cell leukemia DNA vaccine PML-RAP Alpha-hGM-CSF, i.e. the fusion gene pIRES-PMI-RAR Alpha- hGM-CSF. The invention also discloses that the DNA vaccine is applicable in preparing medicine for curing acute promyelocytic leukemia cell leukemia. The invention uses the PML-RAP Alpha fusion gene to construct the DNA vaccine for eradicating the MRD of APL. The invention provides a PML-RAP Alpha-hGM-CSF double-gene DNA vaccine capable of inducing to produce specific anti-APL immune response, which provides important data for research and development of the DNA vaccine for curing APL. The method of the invention can also provide reference for research on other curative tumor DNA vaccines. The DNA vaccine of the invention can clear the minimal residual diseases in the body of APL patients, and finally cure the APL.
Owner:JINAN UNIVERSITY
Anti-tumor DNA vaccine
InactiveUS20160058856A1Improve survivalInhibit growthTumor rejection antigen precursorsAntibody mimetics/scaffoldsFhit geneWilms' tumor
The present invention provides a pharmaceutical composition for treating a tumor, which is a micelle encapsulating at least one tumor-associated antigen gene. The present invention also provides a method for treating a tumor, comprising administering a micelle encapsulating at least one tumor-associated antigen gene to a patient in need of such treatment.
Owner:KYUSHU UNIV
Primer combination for monitoring free tumor DNA, kit and application
InactiveCN108893538ADetection method changesReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceNeoplasm DNA
The invention provides a primer combination for monitoring free tumor DNA, a kit and application. The primer combination is composed of one or two of a first primer pair and a second primer pair, thefirst primer pair is composed of an upstream primer with a base sequence shown as SEQ ID No.1 and a dowmstream primer with a base sequence shown as SEQ ID No.2, and the second primer pair is composedof an upstream primer with a base sequence shown as SEQ ID No.3 and a dowmstream primer with a base sequence shown as SEQ ID No.4. The kit comprises the primer combination. The kit has the advantagesof being quick, simple, convenient, high in specificity, sensitivity and reliability, low in detection cost, short in time, high in detection efficiency and accuracy and low false positive rate.
Owner:邹畅 +3
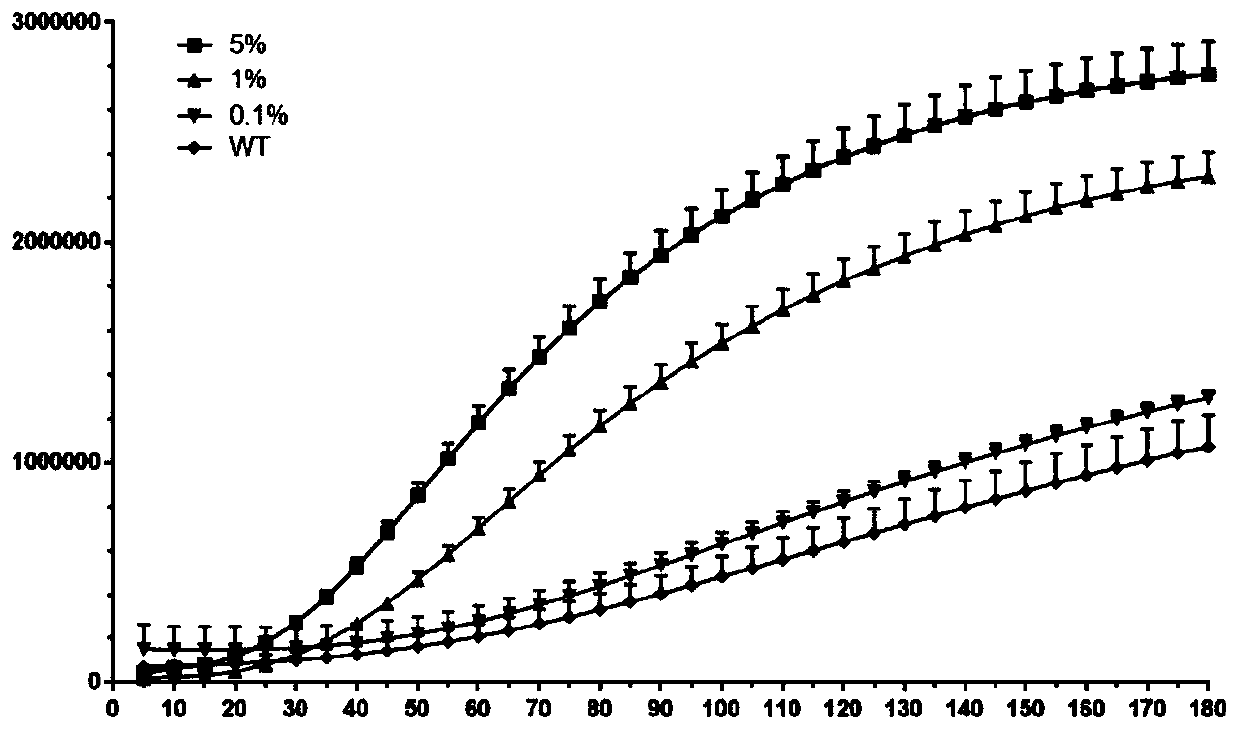
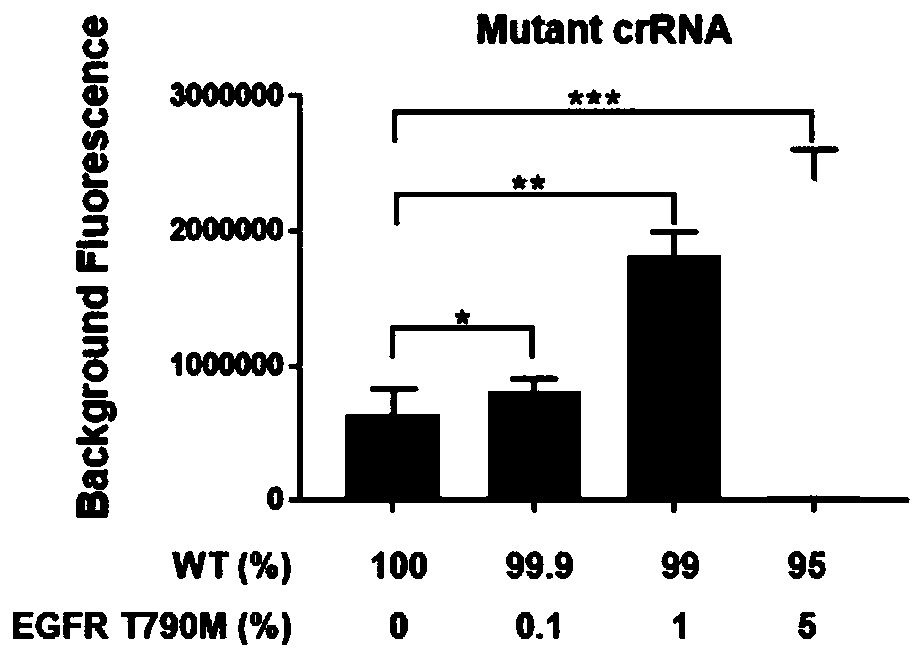
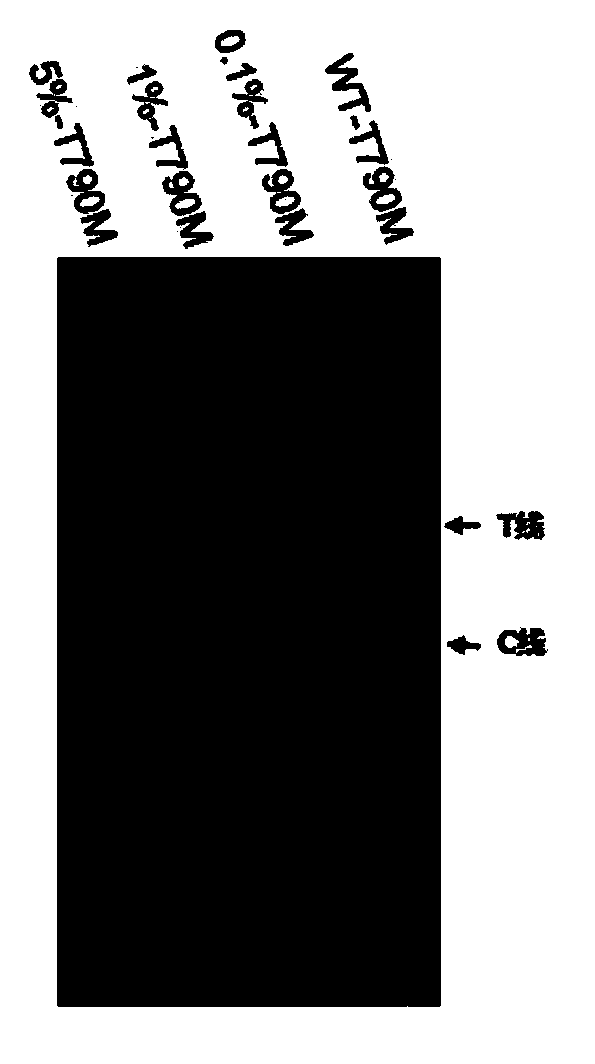
Kit and detection method for detecting EGFR gene T790M mutation
InactiveCN110616261AFast readShorten detection timeMicrobiological testing/measurementMutation frequencyCirculating tumor DNA
The invention provides a kit for detecting EGFR gene T790M mutation and a gene mutation detection method based on the kit, and relates to the technical field of gene detection. The kit comprises an isothermal amplification reaction system, a detection reaction system and at least two circulating tumor DNA standards with known mutation frequencies of an EGFR gene T790M. The kit has the characteristics of being high in sensitivity, high in specificity, simple in operation, easy to carry and the like, and is very suitable for detection of low-frequency mutations in tumor DNA molecules. The detection sensitivity of the detection method based on the kit can reach 10-18 moles per liter, that is the mole level, and the specificity can be achieved by identifying the differences in nucleic acid fragments of a single basic group.
Owner:张鸿
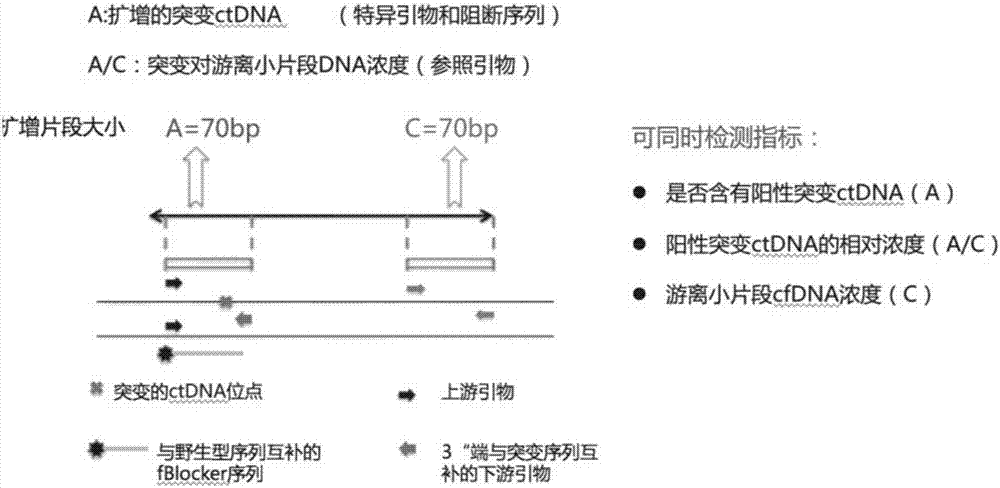
Blood circulating tumor DNA point mutation detection method
InactiveCN107287309AIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationWild typeCirculating tumor DNA
The invention discloses a blood circulating tumor DNA point mutation detection method. The provided complete set of primers comprise a specific primer with an amplification mutation type target sequence and a blockage sequence, wherein the mutation type target sequence is an area, containing mutant sites, of mutation type cfDNA, the specific primer with the amplification mutation type target sequence comprises a mutation type upstream primer and a mutation type downstream primer which are both bonded with the mutation type target sequence; the last basic group at the 3' tail end of the mutation type downstream primer and a mutant basic group located at a mutation site in the mutation type target sequence are complementary; the blockage sequence and a segment containing wild basic groups in a wild target sequence are complementary. The method has the advantages of being high in sensitivity, high in specificity, short in time consumption and low in cost, and multiple indexes can be detected simultaneously.
Owner:罗保君
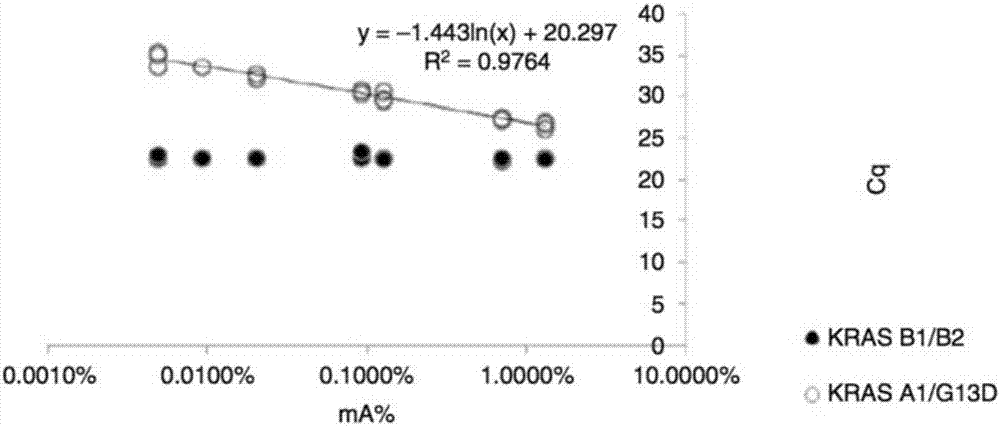
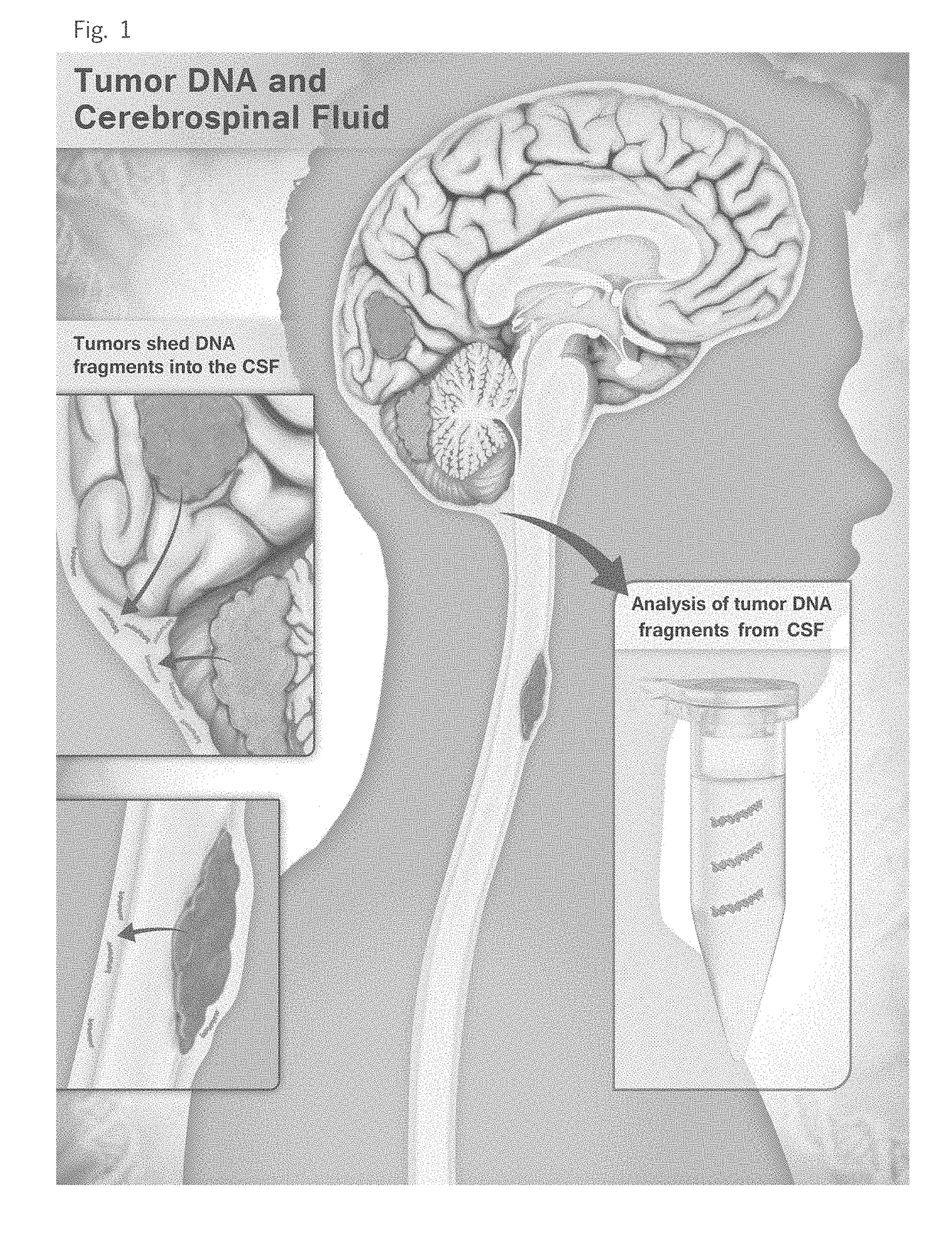
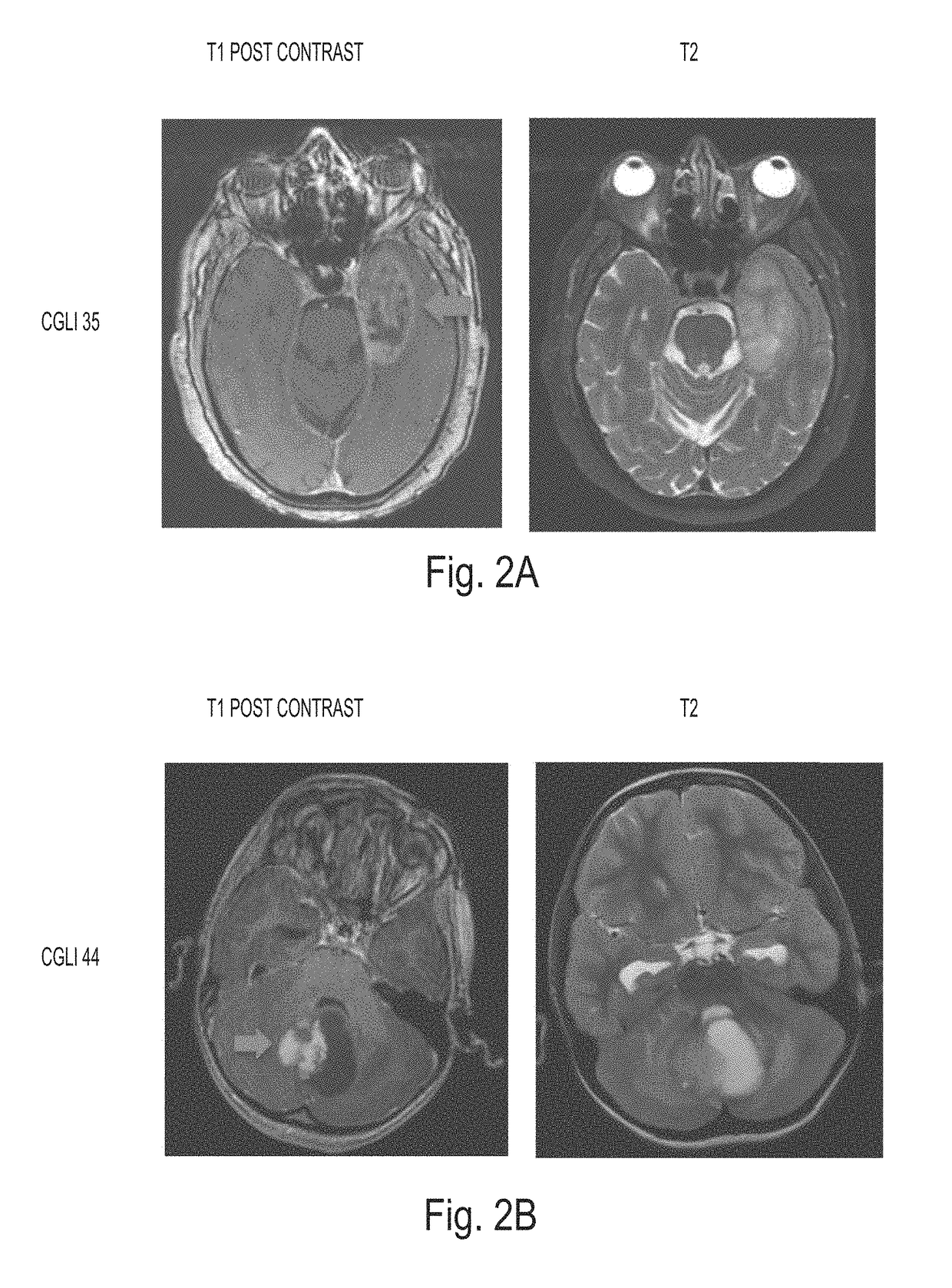
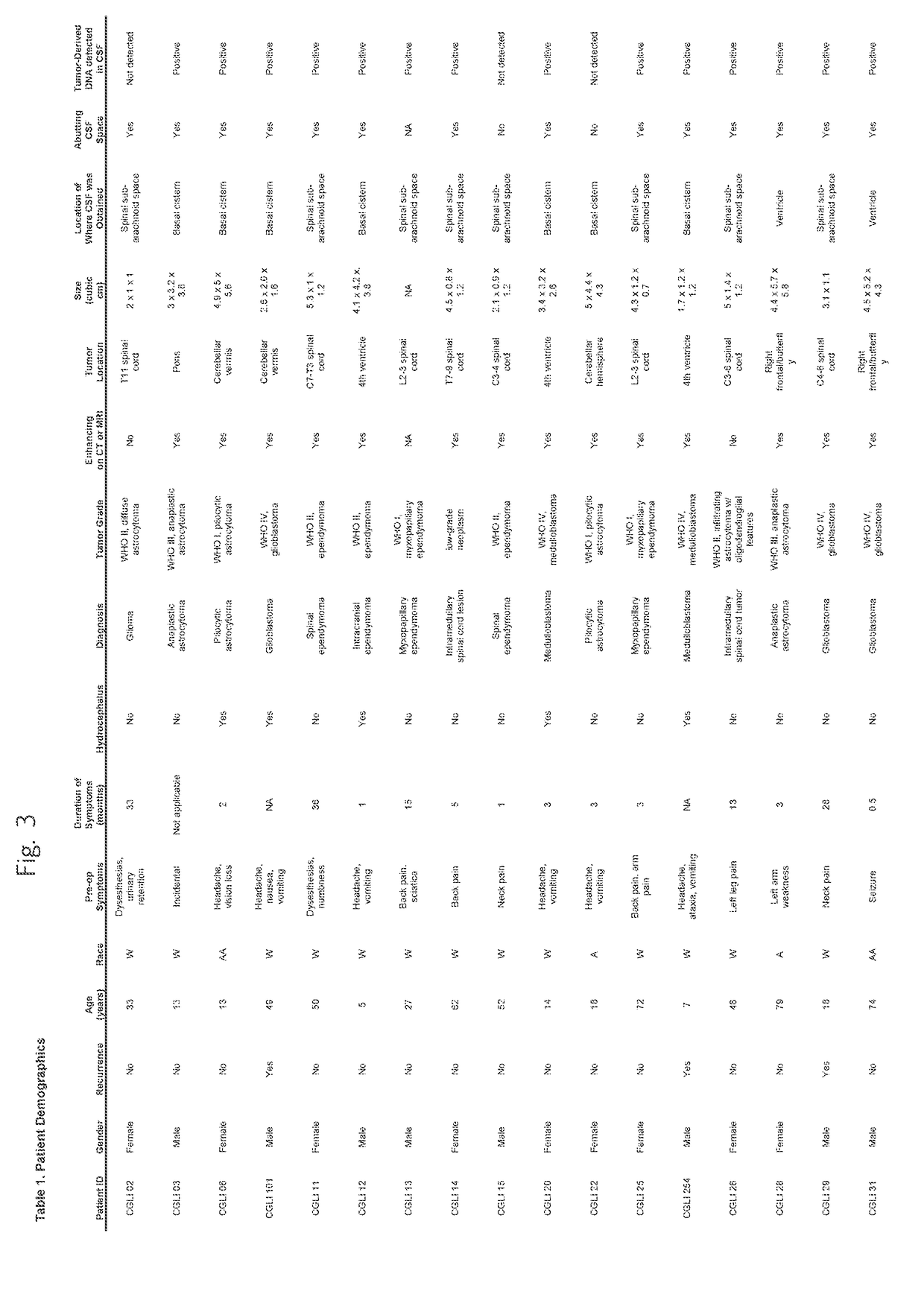
Detection of tumor-derived DNA in cerebrospinal fluid
As cell-free DNA from brain and spinal cord tumors cannot usually be detected in the blood, we assessed the cerebrospinal fluid (CSF) that bathes the CNS for tumor DNA, here termed CSF-tDNA. The results suggest that CSF-tDNA could be useful for the management of patients with primary tumors of the brain or spinal cord.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Mini-intronic plasmid DNA vaccines in combination with LAG3 blockade
ActiveUS9827308B2Less-effective tumor treatmentHigh expressionNucleic acid vectorAntibody ingredientsInteinPlasmid dna
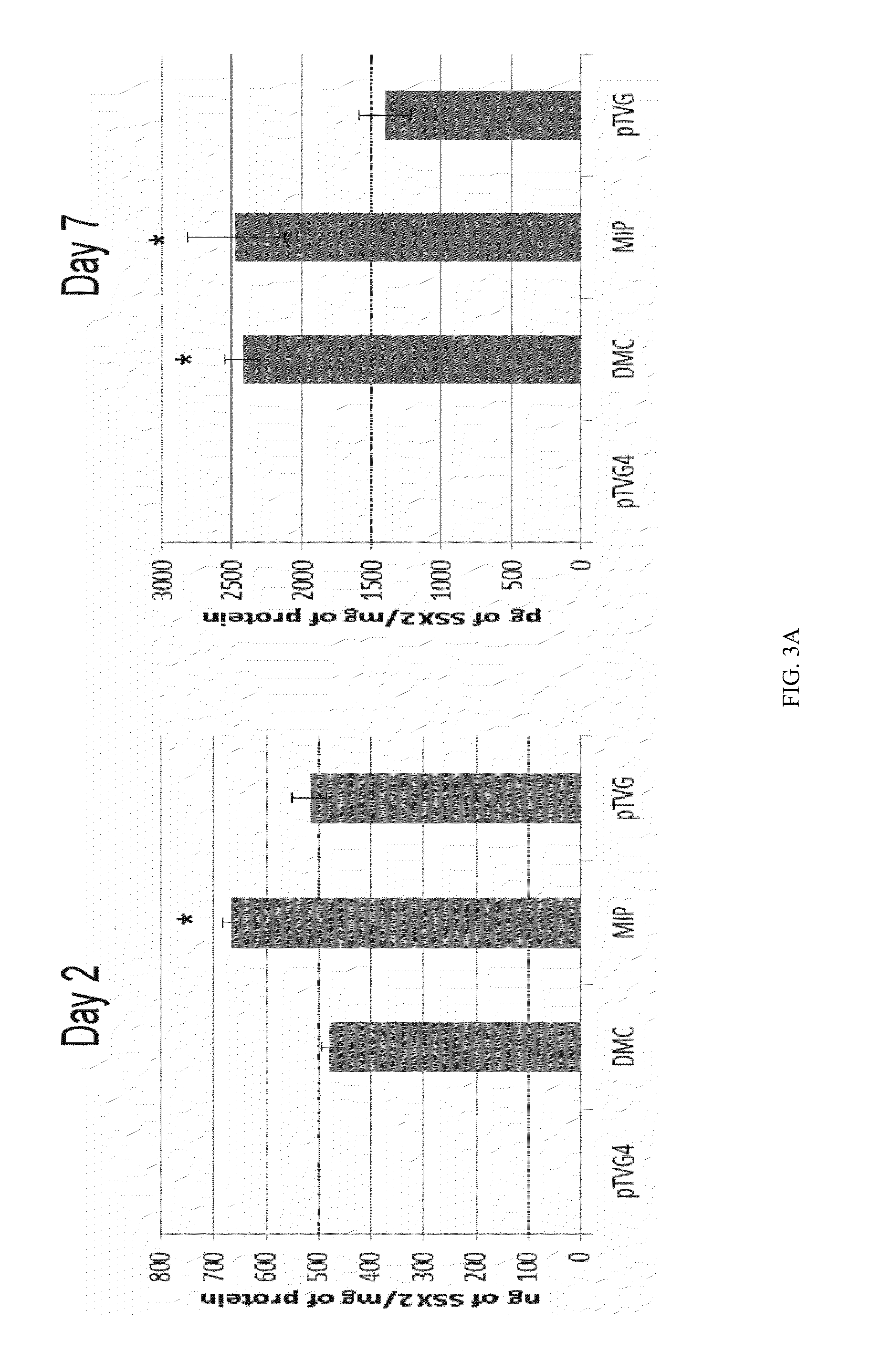
It is disclosed herein that (a) an anti-tumor DNA vaccine delivered using a MIP DNA vector is a less effective tumor treatment than the corresponding anti-tumor DNA vaccine delivered using a conventional pDNA vector, despite the MIP DNA vector eliciting a higher frequency of antigen-specific CD8+ T cells; and (b) tumor infiltrating CD8+ T cells in animals immunized with the MIP DNA vector express higher levels of the immune checkpoint protein LAG-3 than animals immunized with a conventional pDNA vector, while the expression levels of other immune checkpoint proteins was the same for both groups. Based on these findings, improved methods and compositions for administering DNA vaccines are disclosed. Specifically, DNA vaccines delivered with MIP DNA are administered along with a LAG-3 pathway blocking agent, resulting in a more effective vaccine-induced cellular immune response.
Owner:WISCONSIN ALUMNI RES FOUND
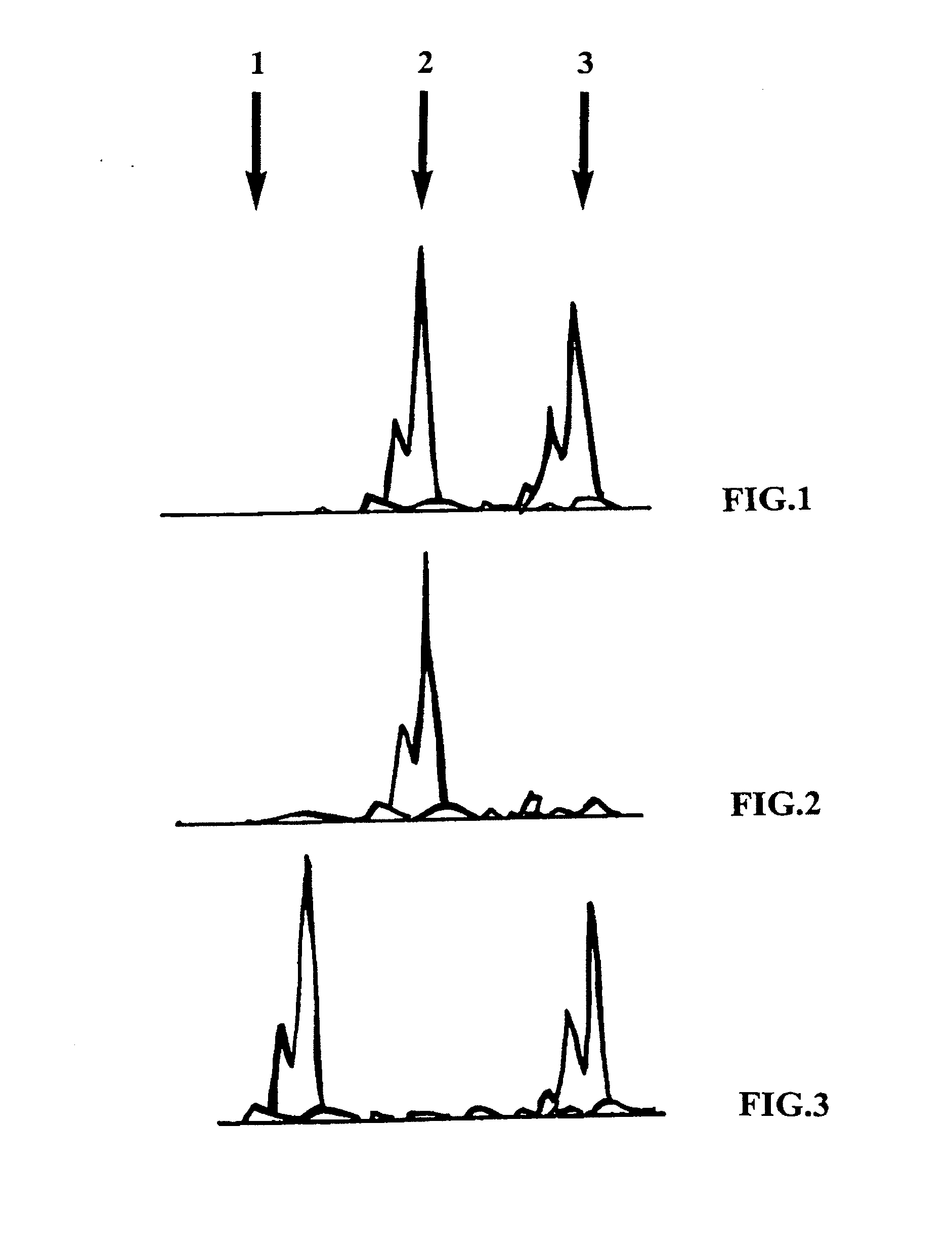
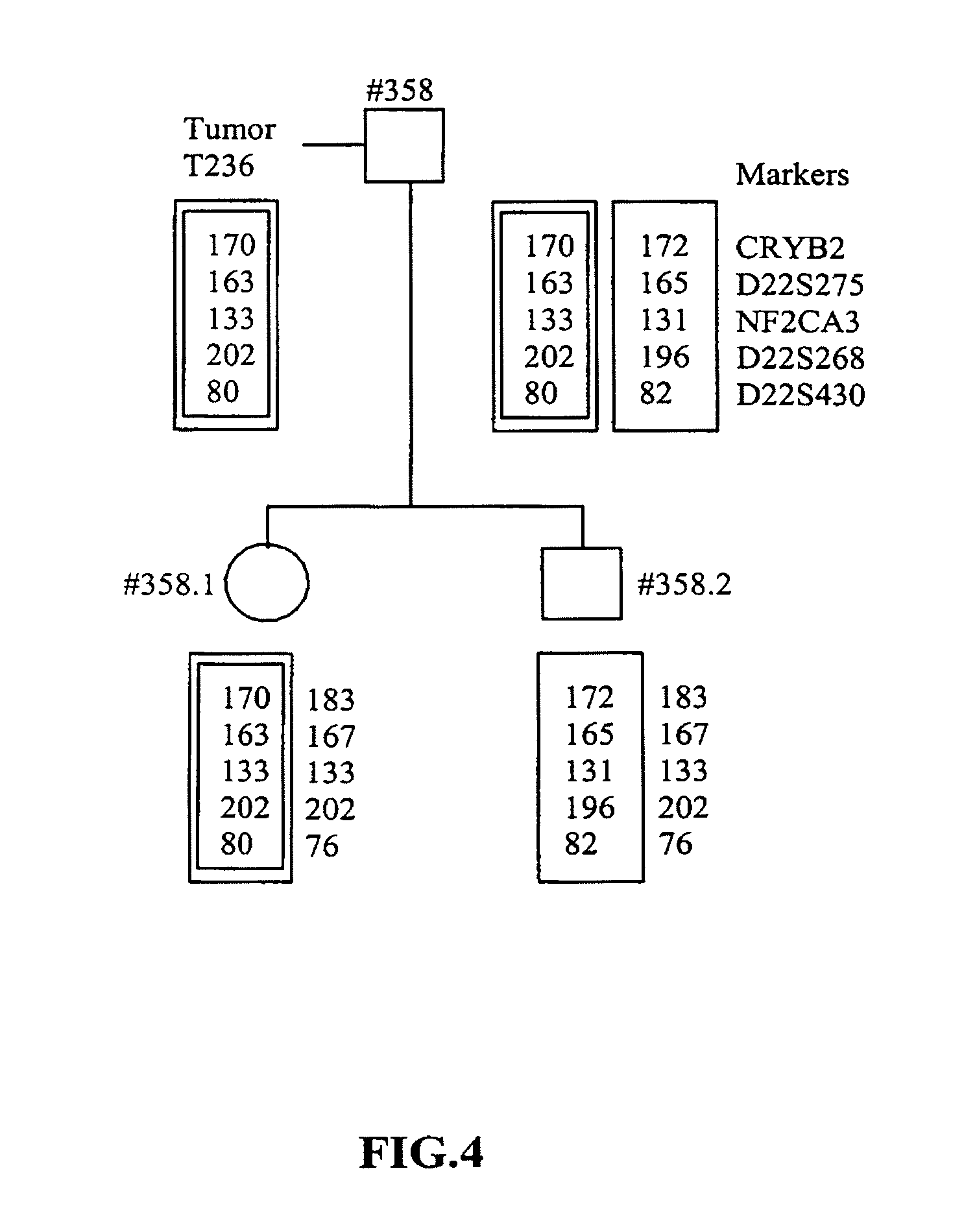
Method for the determination of data for the preparation of the diagnosis of phakomatosis
InactiveUS20110008773A1High data reliabilityImprove reliabilityMicrobiological testing/measurementDiseaseAllele
The invention concerns a method for the determination of data for the preparation of presymptomatic or prenatal diagnosis of phakomatosis, in particular, a tumor suppressor gene disease, in a high-risk patient, in particular of neurofibromatosis, comprising the steps of: making available the tumor material from a person afflicted with the tumor suppressor gene disease, who is a relative of the high-risk patient; isolating tumor DNA from the tumor in the relative; isolating blood DNA from the blood of the relative; amplifying polymorphous DNA microsatellite markers from the tumor and the blood; separating the markers by length; observing the lengths of the markers; comparing the markers from the blood and the tumor; examining for a loss of alleles; optionally, comparing amplified markers from a second tumor of the relative; and amplifying polymorphous DNA microsatellite markers from the blood of an offspring and separating and observing the markers.
Owner:KLUWE LAN
Tumor DNA vaccine and virus vector vaccine taking mucoprotein 1 and surviving as target spots
ActiveCN104491852AImprove immunityImprove tumor inhibition rateGenetic material ingredientsAntibody medical ingredientsVector vaccineViral vector
The invention relates to the field of tumor DNA vaccines and virus vector vaccines. Specifically, the invention relates to a recombinant DNA vector VR-S8 vaccine, a VR-MS vaccine, a recombinant adenovirus Ad-S8 vaccine, an Ad-MS vaccine and a recombinant poxvirus MVA-MS vaccine, and application of the DNA vaccines and the virus vector vaccines as well as the application of optimal combination of immunization ways of the virus vector vaccines in preparation of antitumor vaccines.
Owner:CHANGCHUN BCHT BIOTECH +1
Liquid phase nanometer colloidal gold colorimetric method for quickly detecting malignant tumor DNA (deoxyribonucleic acid)
The invention discloses a liquid phase nanometer colloidal gold colorimetric method for quickly detecting malignant tumor DNA (deoxyribonucleic acid), and belongs to the technical field of malignant tumor detection methods. The method has the technical characteristics that a glass container used for an experiment is cleaned with detergent, after the glass container finishes being cleaned with thedetergent, the glass container is cleaned with distilled water, then, the glass container is dipped in silicified reagent overnight, the inner surface of a gold manufacture vessel is subjected to silicified processing, and the glass container is cleaned with distilled water for standby; 1%(w / v) chloroauric acid and 1%(w / v) sodium citrate solution are prepared, and a filter membrane of which the aperture is 0.2 micrometer is used for filtering; and one magnetic force stirrer is put in a 1000mL of round-bottom flask which is cleaned, and 1000mL of ultrapure water is added. By use of the method,according to the color change of nanometer colloidal gold solution, whether DNA methylation is normal or not can be represented so as to bring convenience for medical personnel in making an early diagnosis for the malignant tumor before clinical symptoms appear, and therefore, the method is favorable for promotion and use.
Owner:吉林省爱诺德生物工程有限公司
Anti-tumor DNA vaccine and preparation method thereof
InactiveCN101485888AImproving immunogenicitySolve the problem of immune toleranceGenetic material ingredientsAntineoplastic agentsBacteroidesAntigen
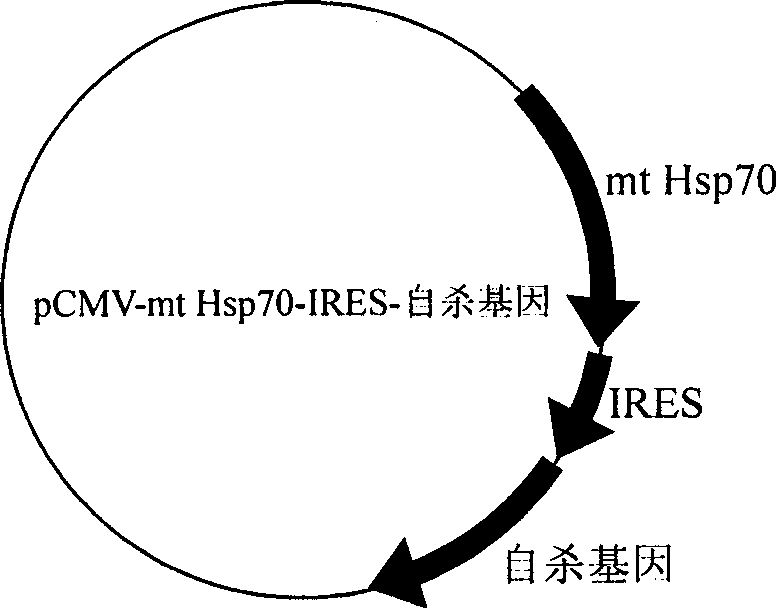
The invention provides an anti-tumor DNA vaccine, which is obtained by transforming pCMV-mt Hsp70-IRES- suicide genes into amphimicrobion which is friendly to human body, anaerobic bacteria which are friendly to the human body, amphimicrobion for reducing toxicity or obligate aerobes for reducing toxicity. The pCMV-mt Hsp70-IRES- suicide genes are recombinant plasmids which are formed by taking pCMV plasmids as an expression vector and tubercle bacillus heat shock protein 70 proteins and suicide genes as antigen genes, wherein the tubercle bacillus heat shock protein 70 proteins and the suicide genes are positioned on the upstream and the downstream of internal ribosome access sites in the pCMV plasmids respectively. The DNA vaccine is used for treating solid tumor and applied through local injection.
Owner:SOUTHERN MEDICAL UNIVERSITY
Tumor DNA vaccine taking mucin 1 and survivin as targets and viral vector vaccine
ActiveCN102086453BImprove immunityImprove tumor inhibition rateGenetic material ingredientsMicroorganism based processesVector vaccineViral vector
The invention discloses a tumor deoxyribonucleic acid (DNA) vaccine taking mucin 1 and survivin as targets and a viral vector vaccine. The invention relates to the field of tumor DNA vaccines and viral vector vaccines, in particular to recombinant DNA vector VR-S8 and VR-MS, recombinant adenovirus Ad-S8 and Ad-MS, and recombinant poxvirus MVA-MS vaccines, and application of the optimum combination of immune ways between DNA vaccines and viral vector vaccines and between the viral vector vaccines in preparing antitumor vaccines.
Owner:CHANGCHUN BCHT BIOTECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com