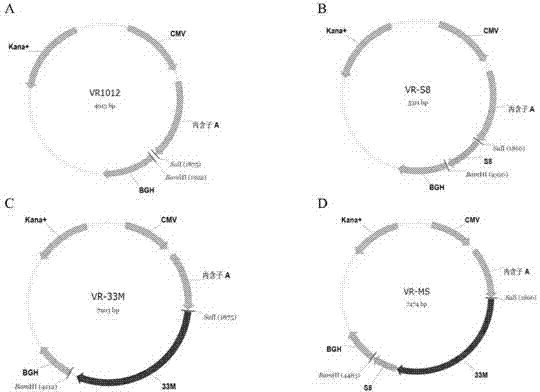
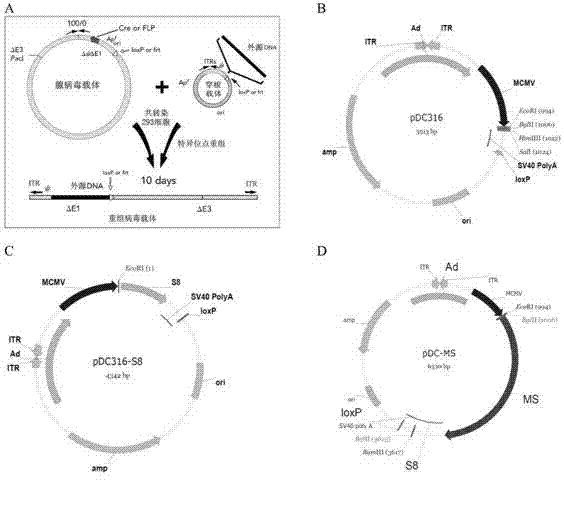
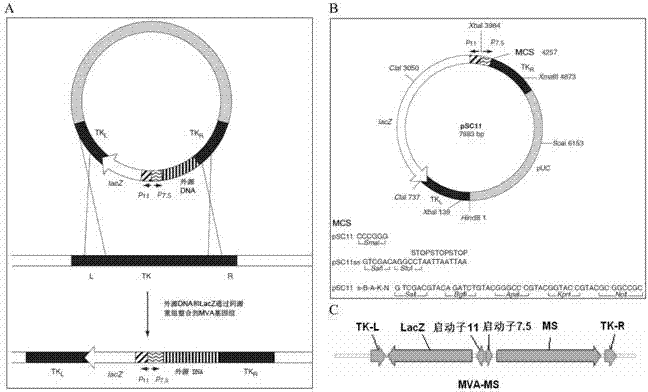
Tumor DNA vaccine and virus vector vaccine taking mucoprotein 1 and surviving as target spots
A technology of DNA vaccine and vaccinia virus, which is applied in the field of tumor DNA vaccine and virus vector vaccine, can solve the problems of relatively large safety hazards in the primary vaccination of recombinant poxvirus vaccine, weak immunogenicity of DNA vector vaccine, and unsatisfactory clinical effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] 1. The preparation method and conditions of the above-mentioned fragment 33M and S8
[0034] Preparation of 1.33M
[0035] MUC1VNTR is a repeat fragment containing 60 bases, GenBank number is NM_002456 (SEQ ID NO: 1). Two pairs of primers were designed according to the base sequence of a VNTR, and a VNTR (1m) repeat region fragment was synthesized by overlapping extension PCR (SOE PCR), and then digested with restriction endonucleases SalI and XhoI to produce the same sticky end characteristics, sequentially connected to construct 33M gene fragments. The specific method is as follows:
[0036] 1) Overlap extension PCR technique
[0037] The PCR reaction conditions are: 95°C for 20s, 55°C for 20s, 72°C for 30s, 30 cycles, using primers P1 (SEQ ID NO:2) and P2 (SEQ ID NO:3) to amplify one copy of MUC1VNTR without ATG (abbreviated as m), using P2 and P3 (SEQ ID NO: 4) as primers to amplify one copy of ATG-containing MUC1VNTR (abbreviated as Am) fragment.
[0038] 2) C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com