Acute progranulocyte leukemia DNA vaccine PML-RAR alpha 384-hIL-2 and preparing method and application thereof
A technology of promyelocytes and DNA vaccines, applied in the field of hematological tumor immunotherapy, can solve problems such as the construction of fusion genes that have not yet been established
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
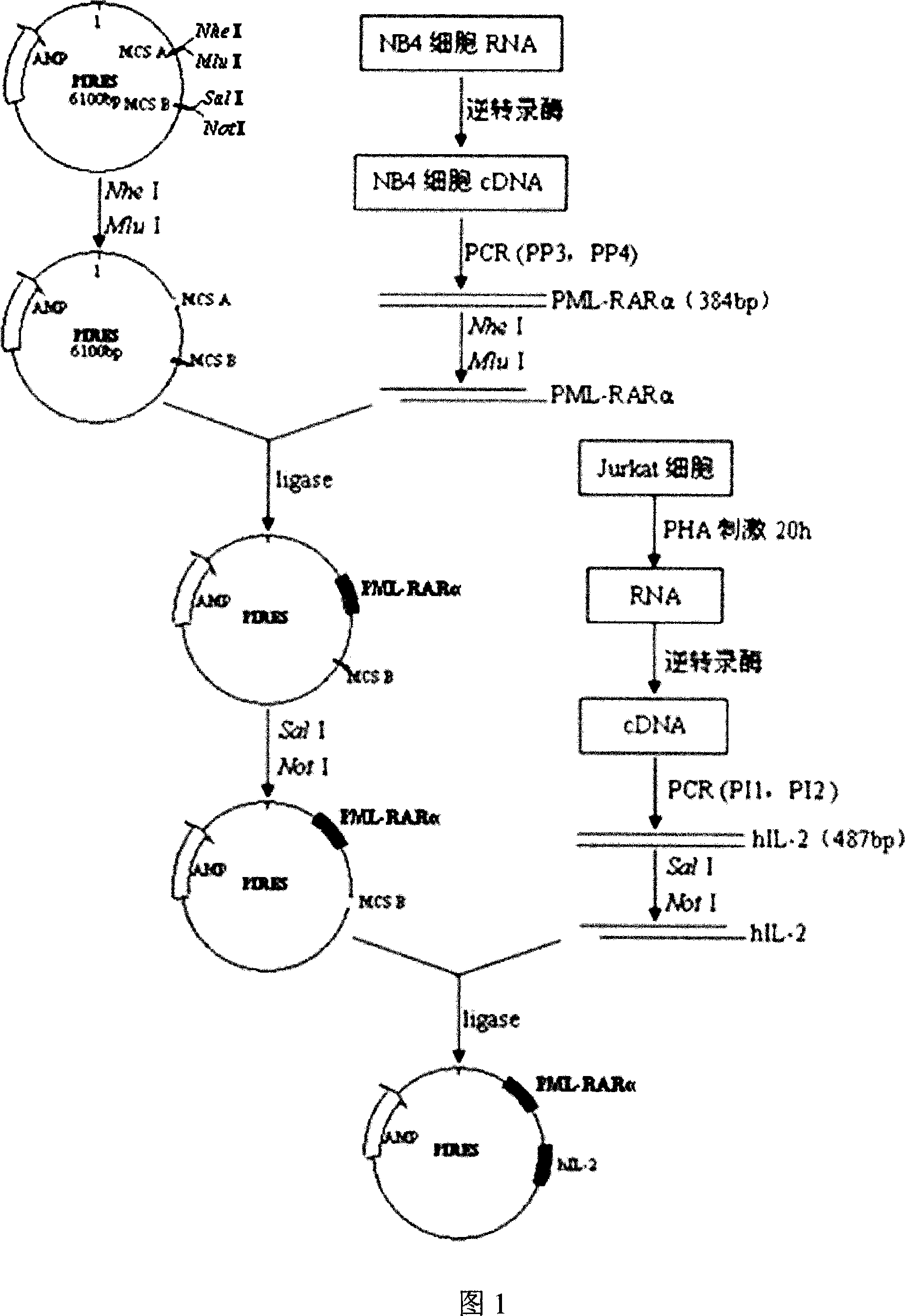
[0085] Example 1 Construction of Acute Promyelocytic Leukemia DNA Vaccine (pIRES-PML-RARα(384bp)-hIL-2 Recombinant Plasmid)
[0086] The construction flow chart is shown in Figure 1, using RT-PCR to amplify the PML-RARα gene, inserting the gene into the multi-cloning site A of the pIRES plasmid to construct pIRES-PML-RARα (384bp), and then by PCR from Jurkat The hIL-2 gene was amplified from the cell cDNA, and the gene was inserted into the multi-cloning site B of pIRES-PML-RARα (384bp) to construct pIRES-PML-RARα (384bp)-hIL-2. After the sequence was correct, PML-RARα384-hIL-2, namely pIRES-PML-RARα(384bp)-hIL-2 plasmid, was successfully constructed.
[0087] Specific steps are as follows:
[0088] 1 Construction of pIRES-PML-RARα recombinant plasmid
[0089] 1.1 Amplification of PML-RARα gene
[0090] Extract RNA from NB4 cell line and synthesize cDNA by reverse transcription as a template (RNA extraction and cDNA synthesis of NB4 cells are carried out according to conven...
Embodiment 2
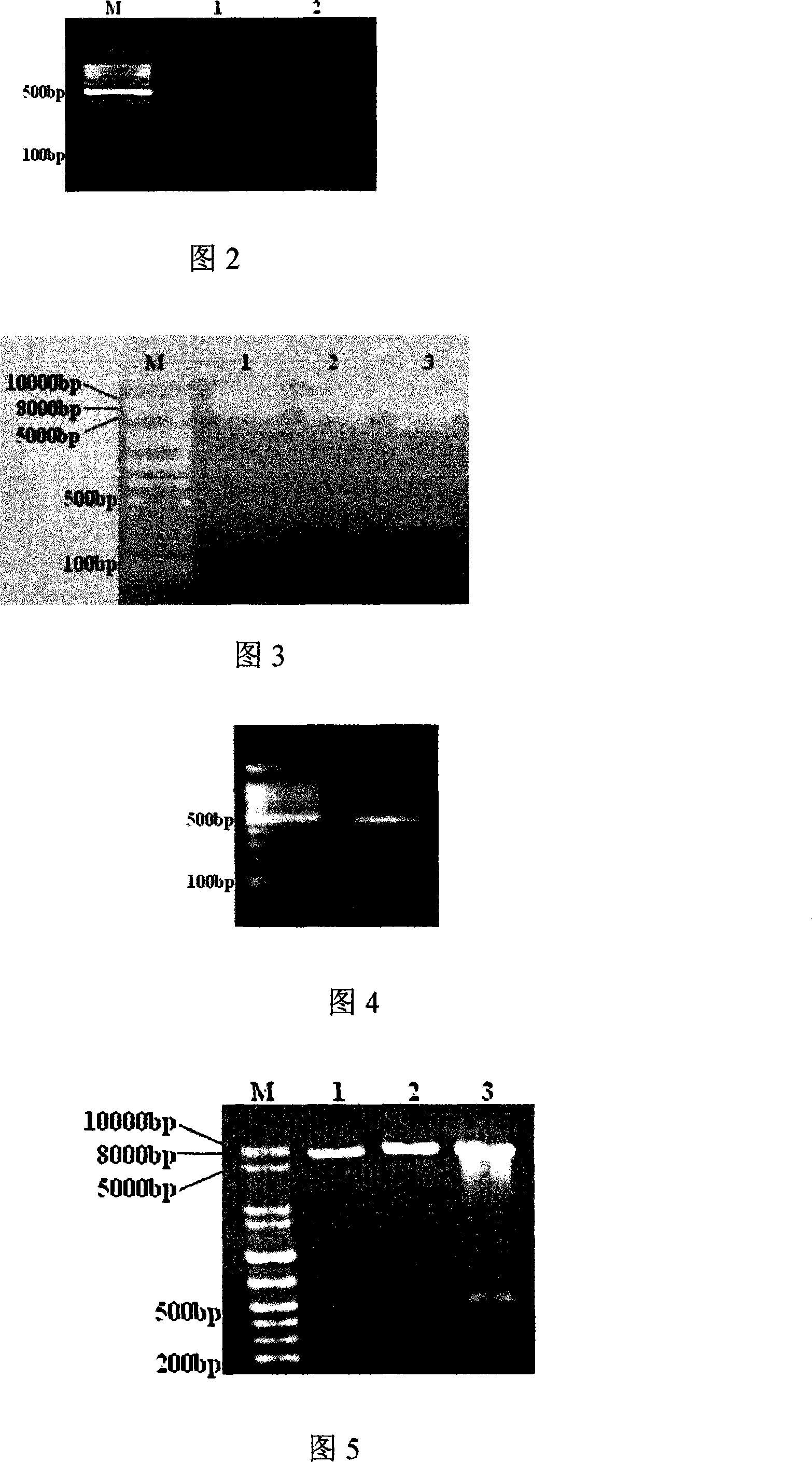
[0124] Example 2 Study on gene and protein expression after transfection of eukaryotic cells by the constructed acute promyelocytic leukemia DNA vaccine (i.e. pIRES-PML-RARα(384bp)-hIL-2 recombinant plasmid)
[0125] 1. Select suitable cells to be transfected. Since the transfection efficiency of suspension cells is low, in order to further improve the transfection efficiency, we use adherent cells for transfection. Using Lipofectamine TM 2000 Kit was transfected into A549 cells. It was proved by nested PCR that the total RNA of A549 cells did not contain hIL-2 gene and PML-RARα gene. A549 cell line was infected. Using Lipofectamine TM 2000Kit transfected A549 cells, put the culture plate into 5% CO 2 After culturing in an incubator (37° C.), the medium was replaced with a medium containing fetal calf serum and antibiotics after 4 to 6 hours, and the supernatant and cells were collected after 48 hours.
[0126] 2 RT-PCR detection
[0127] A549 cells cultured for 48 hours...
Embodiment 3
[0141] Example 3 Study of gene and protein expression, antibody production and specific CTL effect at different stages after mice were injected with acute promyelocytic leukemia DNA vaccine (pIRES-PML-RARα(384bp)-hIL-2 recombinant plasmid)
[0142] Thirty healthy pure-line male BALB / c mice of 6-8 weeks were randomly divided into 5 groups (6 mice in each group). Second-rate. Group A: intramuscular injection of pIRES 200 μg each time into bilateral quadriceps; group B: intramuscular injection of PML-RARα-pIRES 200 μg each time into bilateral quadriceps; PML-RARα-hIL-2-pIRES 200 μg was injected intramuscularly into the quadriceps femoris respectively; Group D: 200 μg hIL-2-pIRES was injected into the quadriceps femoris each time; Group E: each time Intramuscularly inject 200 μg of normal saline into the quadriceps femoris. On the 7th day after the second immunization and the last immunization, 3 mice in each group were randomly selected and sacrificed, and the tibialis anterior...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com