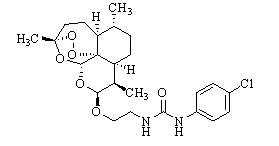
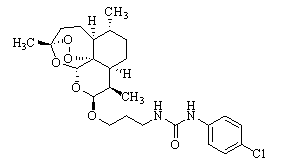
C-10 site carbamido substituted artemisinin derivative, preparation method and application
A kind of derivative, the technology of dihydroartemisinin, applied in the field of medicinal compound and its preparation, can solve the problem of low activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
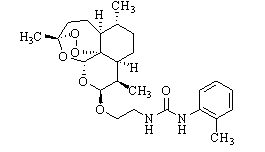
[0157] Experimental example 1: 1-{2-[ (10 R Preparation of )-dihydroartemisinin-10-oxyl]ethyl}-3-(2-methylphenyl)urea
[0158]
[0159] In a 250 ml reaction vial, 4.26 g (15 mmol) of dihydroartemisinin and 3.12 g (15 mmol) of 1-(2-hydroxyethyl)-3-(2-methylphenyl)urea were dissolved in 200 ml In dry dichloromethane, cool to 0°C, add 1mL Et dropwise under stirring 2 O·BF 3 , continued the reaction at 0°C for 8 hours, and monitored the end point of the reaction by TLC to obtain 1-{2-[(10 R )-Dihydroartemisinin-10-oxyl]ethyl}-3-(2-methylphenyl)urea in dichloromethane, with saturated NaHCO 3 solution to quench the reaction, respectively, with saturated NaHCO 3 solution (50 ml×5) and water (50 ml×5) to wash the reaction solution to neutrality, separate the organic layer, and wash with anhydrous Na 2 SO 4 Dry, filter off the desiccant, and distill off the dichloromethane under reduced pressure to obtain the crude product, which is purified by column chromatography (200-300...
experiment example 2
[0160] Experimental example 2: 1-{2-[ (10 R Preparation of )-dihydroartemisinin-10-oxyl]ethyl}-3-(3-chloro-2-methylphenyl)urea
[0161]
[0162] In a 250 ml reaction vial, 4.26 g (15 mmol) of dihydroartemisinin and 3.43 g (15 mmol) of 1-(2-hydroxyethyl)-3-(3-chloro-2-methylphenyl)urea Dissolve in 200 ml of dry dichloromethane, cool to 0°C, add 1 mL of Et dropwise under stirring 2 O·BF 3 , continued the reaction at 0°C for 8 hours, and monitored the end point of the reaction by TLC to obtain 1-{2-[(10 R )-Dihydroartemisinin-10-oxyl]ethyl}-3-(3-chloro-2-methylphenyl)urea in dichloromethane solution with saturated NaHCO 3 solution to quench the reaction, respectively, with saturated NaHCO 3 solution (50 ml×5) and water (50 ml×5) to wash the reaction solution to neutrality, separate the organic layer, and wash with anhydrous Na 2 SO 4 Dry, filter off the desiccant, and distill off the dichloromethane under reduced pressure to obtain the crude product, which is purified ...
experiment example 3
[0164] Experimental example 3: 1-{2-[ (10 R Preparation of )-dihydroartemisinin-10-oxyl]ethyl}-3-phenylurea
[0165]
[0166] In a 250 ml reaction vial, 4.26 g (15 mmol) of dihydroartemisinin and 2.7 g (15 mmol) of 1-(2-hydroxyethyl)-3-phenylurea were dissolved in 200 ml of dry dichloromethane , cooled to 0°C, 1mL Et was added dropwise with stirring 2 O·BF 3 , continued the reaction at 0°C for 8 hours, and monitored the end point of the reaction by TLC to obtain 1-{2-[(10 R )-dihydroartemisinin-10-oxyl]ethyl}-3-phenylurea in dichloromethane, saturated NaHCO 3 solution to quench the reaction, respectively, with saturated NaHCO 3 solution (50 ml×5) and water (50 ml×5) to wash the reaction solution to neutrality, separate the organic layer, and wash with anhydrous Na 2 SO 4 Dry, filter off the desiccant, and distill off dichloromethane under reduced pressure to obtain the crude product, which is purified by column chromatography (200-300 mesh silica gel, petroleum ethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com