Nanoparticle liposome for releasing different medicines in programmed manner and preparation and use thereof
A procedural, nanoparticle technology, applied in liposome delivery, drug combination, medical preparations of non-active ingredients, etc., can solve problems such as undiscovered, cationic nanoparticle injection toxicity, etc., to improve utilization rate and reduce medical treatment Cost, effect of reducing patient suffering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of DMAB modified doxorubicin nanoparticles
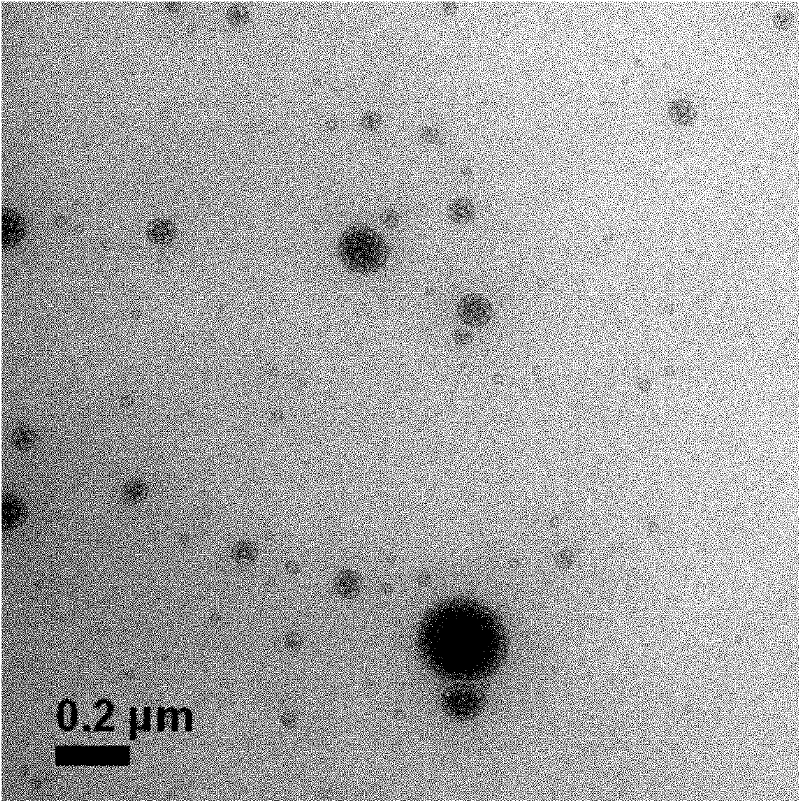
[0040] Get 100mg didodecyl dimethyl ammonium bromide preparation mass concentration and be 0.5% didodecyl dimethyl ammonium bromide aqueous solution 20mL, the mass ratio of lactide and glycolide in polylactic acid glycolic acid copolymer 50:50, 200 mg of poly(lactic-co-glycolic acid) copolymer with a molecular weight of 8000, 10 mg of doxorubicin were dissolved in 10 mL of ethyl acetate, emulsified and homogenized at 7000 r / min for 15 minutes, added distilled water to 100 mL, dispersed the emulsified system, dispersed After rotary evaporation, remove the organic solvent, centrifuge at 30000r / min for 30min, remove excess didodecyldimethylammonium bromide, discard the supernatant, wash with distilled water three times, and freeze-dry to obtain the doxorubicin-loaded nano Capsule 120mg.
Embodiment 2
[0041] Embodiment 2: Preparation of DMAB modified doxorubicin nanoparticles
[0042] Get 200mg of dodecyl dimethyl ammonium bromide preparation concentration and be 1% didodecyl dimethyl ammonium bromide aqueous solution 20mL, the mass ratio of lactide and glycolide in polylactic acid glycolic acid copolymer is 75 : 25, the molecular weight is 60000 poly(lactic-co-glycolic acid) copolymer 300mg, 10mg doxorubicin is dissolved in 10mL acetone, 10000r / min emulsifies and homogenizes for 15 minutes, adds distilled water and settles to 100mL, disperses the emulsified system, rotatively evaporates after dispersion, Remove the organic solvent, centrifuge at 30,000 r / min for 45 minutes, remove excess didodecyldimethylammonium bromide, discard the supernatant, wash with distilled water three times, and freeze-dry to obtain 210 mg of doxorubicin nanoparticles.
Embodiment 3
[0043] Embodiment 3: Preparation of DMAB modified doxorubicin nanoparticles
[0044] Get 800mg didodecyl dimethyl ammonium bromide preparation concentration and be 2% didodecyl dimethyl ammonium bromide aqueous solution 40mL, make the mass ratio of lactide and glycolide in the polylactic acid glycolic acid copolymer 25 : 75, the molecular weight is 100000 poly(lactic-co-glycolic acid) copolymer 2300mg, 10mg doxorubicin is dissolved in 20mL acetone, 15000r / min is emulsified and homogenized for 15 minutes, distilled water is added and the volume is adjusted to 250mL, the emulsified system is dispersed, and rotary evaporation after dispersion, Remove the organic solvent, centrifuge at 60,000 r / min to remove excess didodecyldimethylammonium bromide, discard the supernatant, wash with distilled water three times, and freeze-dry to obtain 1.5 g of doxorubicin nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com