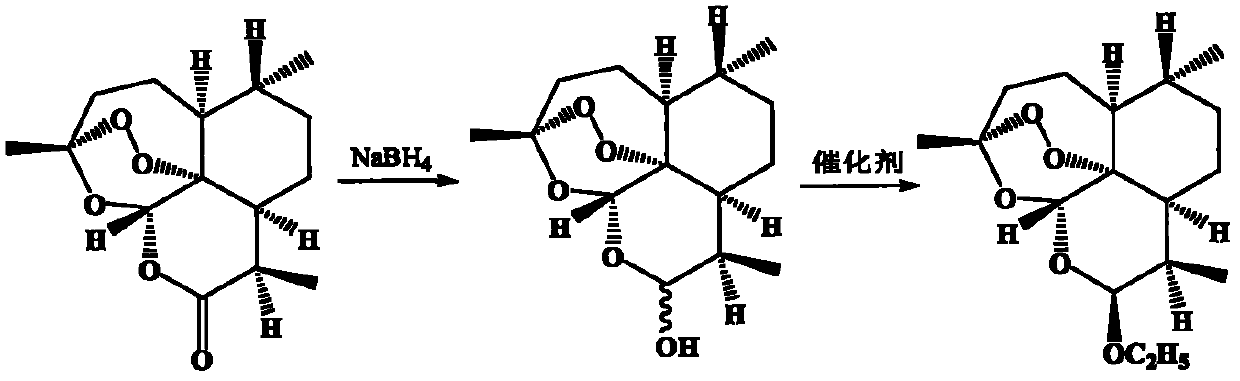
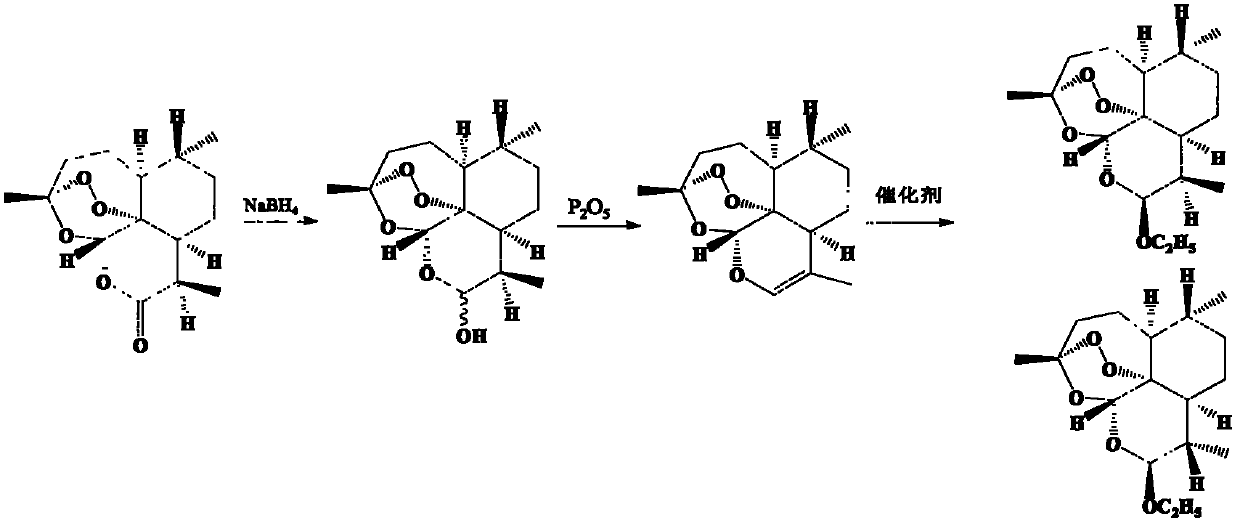
New process for preparing beta-arteether by single reaction kettle method by taking artemisinin as raw material
A technology of artemisinin and arteether, applied in organic chemistry and other directions, can solve the problems of incomplete reaction, residual raw materials, and many technological processes, and achieve the effects of reducing the use of organic solvents, reducing intermediate processes, and improving economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 5.0 kg of artemisinin into a jacketed stainless steel reaction kettle, add 80.0 L of absolute ethanol, stir and dissolve at room temperature. The freezing device controlled the temperature in the stainless steel reactor to 0°C, added 3.5 kg of zinc borohydride in batches within 30 minutes, and then controlled the temperature below 5°C to react for 30 minutes, and monitored by TLC until the raw material point of artemisinin disappeared. Control the temperature in the reaction kettle below 10°C, add methanesulfonic acid or p-toluenesulfonic acid to adjust the pH value to about 7 to terminate the reduction reaction. Continue to drop 0.70kg anhydrous ZnI 2 or 0.50kg anhydrous ZnBr 2 , reacted at 45°C for 2 hours under temperature control, and monitored by TLC until the dihydroartemisinin point disappeared. Control the temperature at 55°C, concentrate under reduced pressure until no solvent is removed, add 50 L of ethyl acetate and 100 L of pure water, and stir and e...
Embodiment 2
[0033] Weigh 5.0 kg of artemisinin into a jacketed stainless steel reaction kettle, add 50.0 L of tetrahydrofuran, stir and dissolve at room temperature. The freezing device controlled the temperature in the stainless steel reactor to 0°C, and added 3.0 kg of potassium borohydride in batches within 60 minutes, and then controlled the temperature below 15°C to react for 60 minutes, and monitored by TLC until the raw material point of artemisinin disappeared. Control the temperature in the reactor below 10°C, add phosphoric acid or adjust the pH value to about 7 with concentrated sulfuric acid to terminate the reduction reaction. Continue to drop into absolute ethanol 10L and 0.60kg anhydrous SnCl 2 , CaCl 2 , FeCl 3 or SbCl 3 , reacted at 55°C for 3 hours under temperature control, and monitored by TLC until the dihydroartemisinin point disappeared. Control the temperature at 45°C, concentrate under reduced pressure until no solvent is removed, add 50 L of dichloroethane an...
Embodiment 3
[0035] Weigh 5.0 kg of artemisinin into a jacketed stainless steel reaction kettle, add 60.0 L of 1,3-dioxane, and stir to dissolve at room temperature. The freezing device controlled the temperature in the stainless steel reactor to 0°C, and added 2.5 kg of lithium borohydride in batches within 40 minutes, and then controlled the temperature below 20°C for 50 minutes, and monitored by TLC until the raw material point of artemisinin disappeared. Control the temperature in the reaction kettle below 10°C, add phosphoric acid or concentrated sulfuric acid to adjust the pH value to about 7 to terminate the reduction reaction. Continue to drop into absolute ethanol 10L and 0.50kg anhydrous (Ph3P) 3 RhCl, temperature controlled at 15°C for 2 hours, TLC monitoring to dihydroartemisinin point 1‰ dihydroartemisinin standard substance point. Control the temperature at 45°C, concentrate under reduced pressure until no solvent is removed, add 50 L of dichloromethane and 100 L of pure wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com