Human replication-deficient recombinant adenovirus vector for efficiently expressing leishmania PEPCK
A technology of recombinant adenovirus and Leishmania, which is applied to viruses/phages, medical preparations containing active ingredients, viruses, etc., can solve the problems of highly weakened pathogenicity and impaired proliferation of Leishmania, and achieve High safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of recombinant adenovirus vector Ad5-L.m-PEPCK:
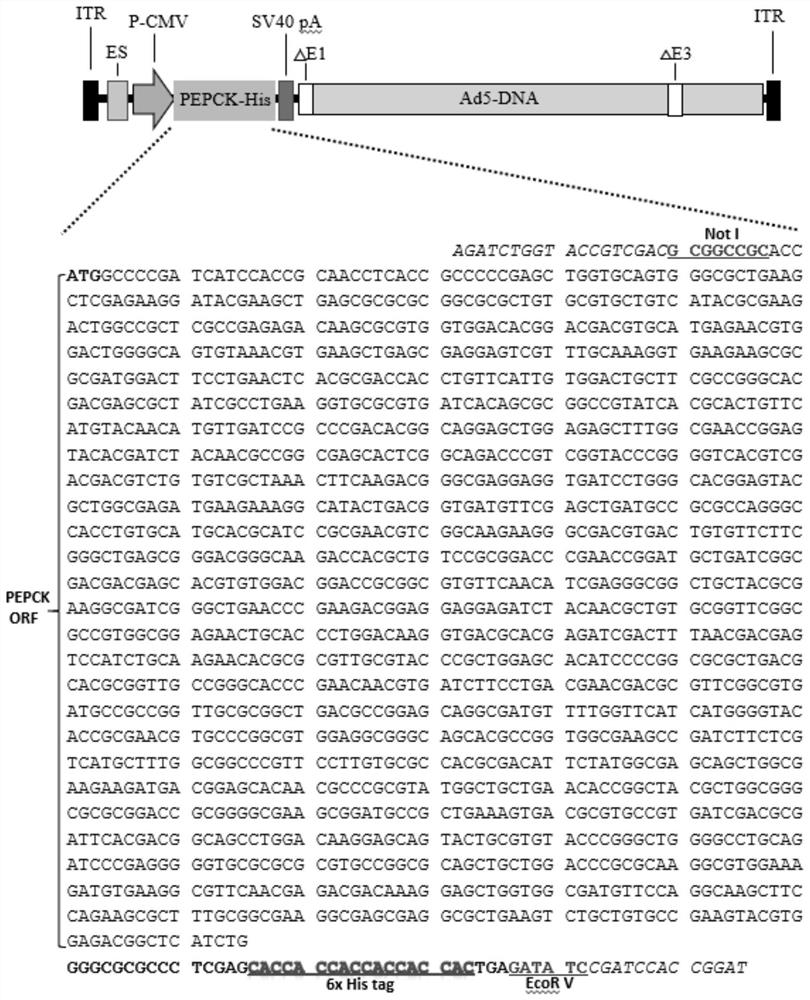
[0039] 1. According to the coding sequence of Leishmania PEPCK gene (NCBI GenBank database entry XM_003721939.1; Leishmania major strain Friedlin) (a total of 1575 nucleotides, nucleotides, encoding 525 amino acids), the carboxyl terminal containing 6x Histidine (6xHis) was synthesized The full-length PEPCK gene. Add a sequence containing a NotI restriction site in front of the start codon ATG GCGGCCGC ACC; add the sequence encoding 6x His before the stop codon TGA, and add the sequence of EcoRV restriction enzyme cutting site behind GATATC ( figure 1 ).
[0040] 2. Add 11 additional modified amino acids GRALEHHHHHH to the carboxyl terminus of the PEPCK gene containing a 6x His tag at the carboxyl terminus, so that the expressed protein contains a total of 536 amino acids.
[0041] 3. By using NotI and EcoRV double enzyme digestion, the above-mentioned modified PEPCK gene and the shuttle vector pShuttle...
Embodiment 2
[0048] Ad5-L.m-PEPCK separation and purification:
[0049] In order to obtain a large amount of purified recombinant adenovirus vectors, 293 cells were infected with the primary vector Ad5-L.m-PEPCK in Example 1, and the multiplicity of infection (MOI) was 1 plaque forming unit (PFU). 48 hours after infection, infected cells were harvested and freeze-thawed 3 times. Cell lysates were subjected to stepwise gradient centrifugation with 1.2 and 1.4 g / ml CsCl and ultracentrifuged at 35,000 rpm for 2 h. Harvesting the main strip ( image 3 The position indicated by the middle arrow), and then dialyzed into the dialysate (100mM sucrose; 10mM Tris-HCl, pH 8.0; 2mM MgCl 2 ), stored at -80°C. Dialysis At 4°C, dialyze for 24 hours, and change the dialysate every 6 hours, 2-3 liters of dialysate each time.
Embodiment 3
[0051] Purification and immunoassay of Ad5-L.m-PEPCK protein expressed in cells (PEPCK):
[0052] 293 cells were infected with Ad5-L.m-PEPCK preserved in Example 2, and the multiplicity of infection (MOI) was 1 plaque forming unit (PFU). After 48 hours of infection, the infected cells were harvested and washed with 4°C phosphate-buffered saline (PBS, 137mM NaCl; 2.7mM KCl; 10mM NaCl 2 HPO 4 ; 1.8mM KH 2 PO 4 ) wash, in containing 50mM NaH 2 PO 4 buffer [pH 7.4, 300 mM NaCl, 10 mM imidazole, 0.5% Triton X-100, 10% glycerol and protease inhibitor cocktail (Roche)], and then centrifuged at 22,000×g for 30 min at 4°C. Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) was added to the clarified supernatant and incubated at 4°C for 2 hours to capture His-tagged proteins. The precipitated protein was eluted with 250 mM imidazole solution, separated on 10% polyacrylamide gel electrophoresis (SDS-PAGE), and stained with Coomassie brilliant blue R250 ( Figure 4 ) and Western blot ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com