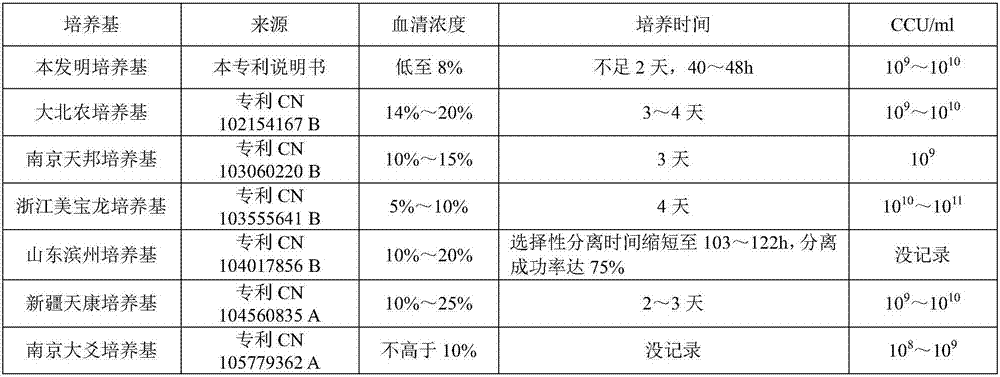
Mycoplasma hyopneumoniae culture medium and preparation method thereof
A technology of Mycoplasma hyopneumoniae and culture medium, which is applied in the field of Mycoplasma hyopneumoniae culture medium and its preparation, can solve the problems of difficult to exceed titer and high serum dosage, and achieve the effects of reducing production costs, reducing pollution risks, and reducing allergic stress reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
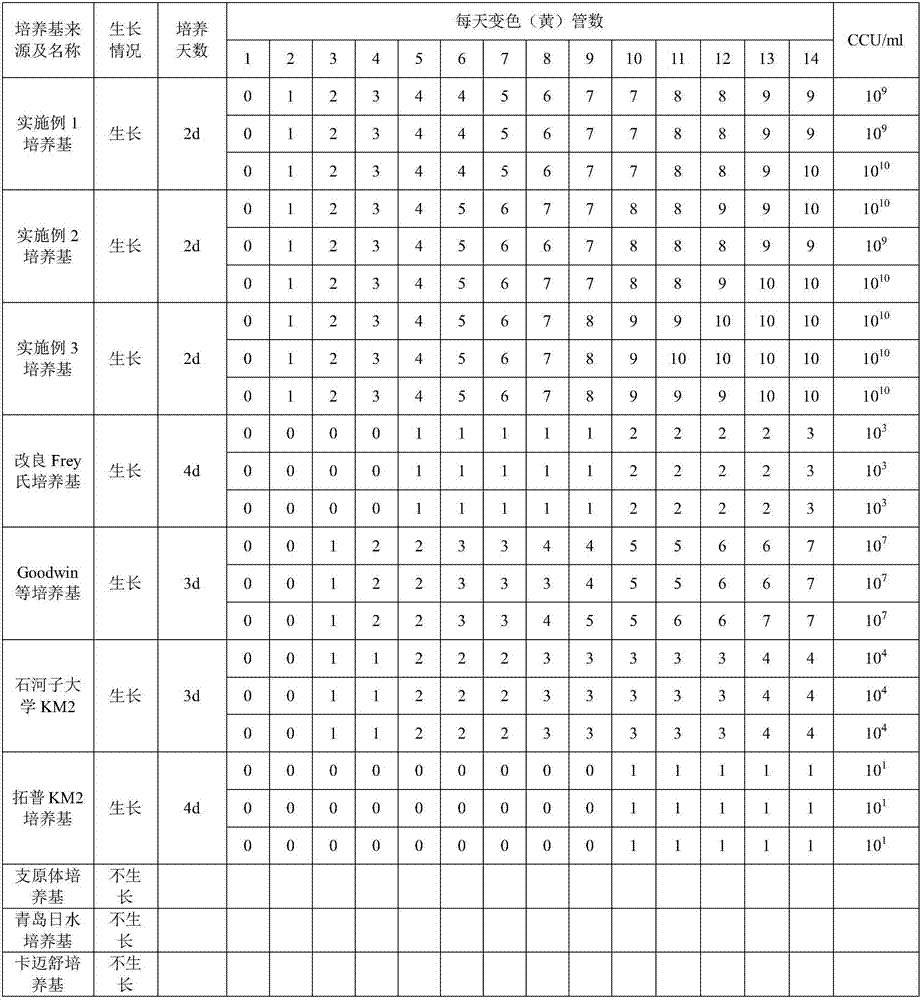
Embodiment 1
[0043] ——Preparation of culture medium of the present invention
[0044] 1. Preparation of 20× Hank's solution:
[0045] 1) 20×Hank's1: Weigh 16g of NaCl, MgSO 4 ·7H 2 O 0.5g, KCl 1.0g, MgCl · ·6H 2 O 0.5g, each component was dissolved in 90ml of deionized water one by one, weighed CaCl 2 Dissolve 0.5g in 10ml deionized water and mix the two;
[0046] 2) 20×Hank's2: Weigh Na 2 HPO 4 12H 2 O 0.4g, KH 2 PO 4 0.35 g and 5 g of glucose were dissolved in 98 ml of deionized water one by one, and 2 ml of 1% phenol red solution was added.
[0047] 2. Preparation of basal medium:
[0048] Measure 20×Hank’s 1 35ml and 20×Hank’s 2 35ml in 722ml deionized water, weigh 2g of yeast extract, 0.5g of milk protein hydrolyzate, and 6g of beef heart extract, stir and dissolve one by one, adjust to 10M NaOH pH to 7.4, autoclave at 115°C for 20min.
[0049] 3. Preparation of auxiliary medium:
[0050] 1) 5% arginine solution: Weigh 5 g of L-arginine and dissolve it in 100 ml of dei...
Embodiment 2
[0056] ——Preparation of culture medium of the present invention
[0057] (1) Preparation of 20× Hank's solution:
[0058] 1) 20×Hank's1: Weigh NaCl 12g, MgSO 4 ·7H 2 O 0.1g, KCl 0.4g, MgCl 2 ·6H 2 O 0.1g, each component was dissolved in 90ml deionized water one by one, weighed CaCl 2 Dissolve 0.2g in 10ml deionized water and mix the two;
[0059] 2) 20×Hank's2: Weigh Na 2 HPO 4 12H 2 O 0.1g, KH 2 PO 4 0.10 g and 5 g of glucose were dissolved in 96 ml of deionized water one by one, and 4 ml of 1% phenol red solution was added.
[0060] (2) Preparation of basal medium:
[0061] Measure 20×Hank’s 1 25ml and 20×Hank’s 2 25ml in 825ml deionized water, weigh 5g of yeast extract, 3g of milk protein hydrolyzate, and 12g of beef heart extract, stir and dissolve one by one, adjust the pH with 10M NaOH To 7.2, autoclave at 115°C for 20 minutes.
[0062] (3) Preparation of auxiliary medium:
[0063] 1) 5% arginine solution: Weigh 5 g of L-arginine and dissolve it in 100 ml...
Embodiment 3
[0069] ——Preparation of culture medium of the present invention
[0070] (1) Preparation of 20× Hank's solution:
[0071] 1) 20×Hank's1: Weigh 15g of NaCl, MgSO 4 ·7H 2 O 0.2g, KCl 0.6g, MgCl 2 ·6H 2 O 0.3g, each component was dissolved in 90ml deionized water one by one, weighed CaCl 2 Dissolve 0.3g in 10ml deionized water, and mix the two;
[0072] 2) 20×Hank's2: Weigh Na 2 HPO 4 12H 2 O 0.2g, KH 2 PO 4 0.20 g and 2 g of glucose were dissolved in 97 ml of deionized water one by one, and 3 ml of 1% phenol red solution was added.
[0073] (2) Preparation of basal medium:
[0074] Measure 20×Hank’s 1 30ml and 20×Hank’s 2 30ml in 780ml deionized water, weigh 3g of yeast extract, 2g of milk protein hydrolyzate, and 8g of beef heart extract, stir and dissolve one by one, adjust the pH with 10M NaOH To 7.3, autoclave at 115°C for 20min.
[0075] (3) Preparation of auxiliary medium:
[0076] 1) 5% arginine solution: Weigh 5 g of L-arginine and dissolve it in 100 ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com