Porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of cell and attenuation via drug screening and application thereof
A porcine pseudorabies and attenuated vaccine technology, which is applied in the application field of porcine pseudorabies virus gene deletion attenuated vaccine strains, prevention and treatment of porcine pseudorabies, can solve the problems that sheep cannot completely resist the attack of PRVHeN1 strain and cannot effectively neutralize the virus , to achieve the effects of differential diagnosis, high protection efficiency and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
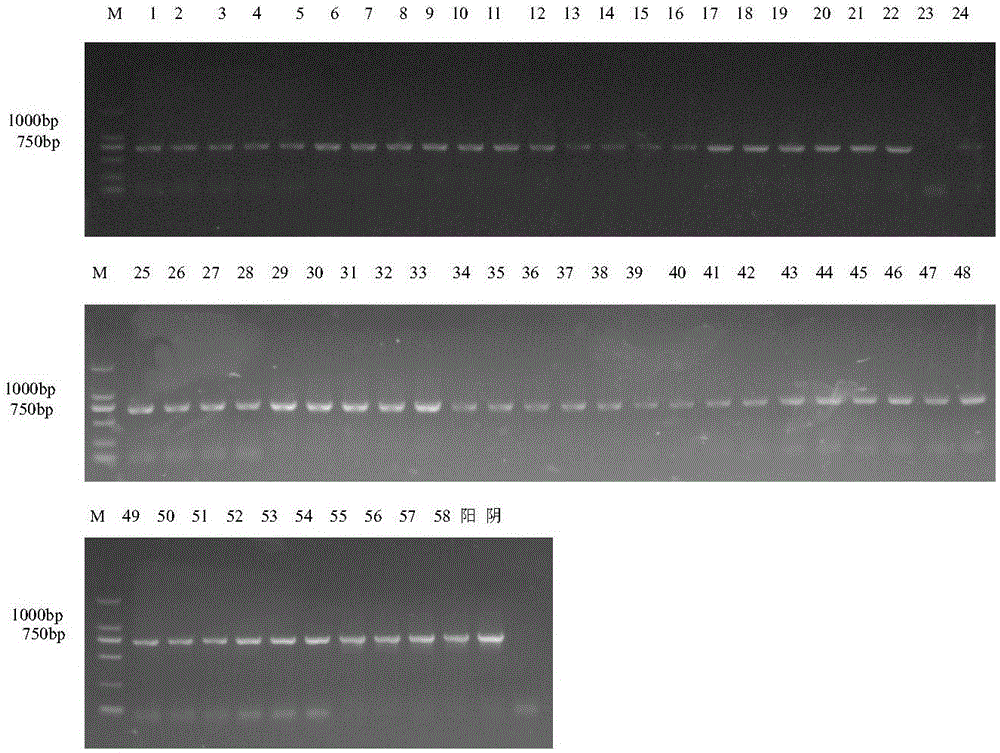
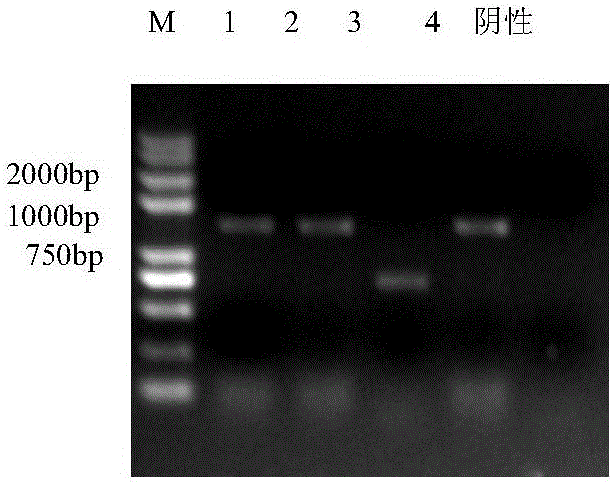
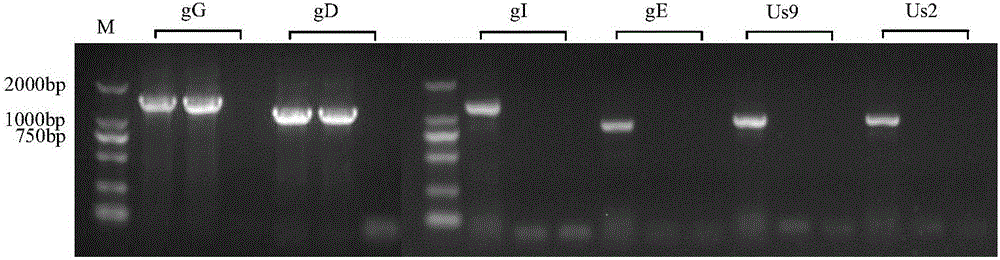
[0028] Embodiment 1, the acquisition and molecular characteristic identification of porcine pseudorabies virus TP strain
[0029] Porcine pseudorabies virus HeN1 strain (this virus strain has been recorded in the patent application with publication number CN102994458, and the invention name is "Porcine pseudorabies virus virulent strain, its gene deletion vaccine strain and its application" has been deposited in China Microorganism General Microbiology Center of Species Preservation Management Committee, Microbial Preservation Number is CGMCC NO.6656.) The F10 generation of Vero cells that grow into a good monolayer is inoculated, the temperature of the cell incubator is adjusted to 35 ° C, and the virus is harvested after 2-3 days of cultivation. Freeze and thaw once to continue passage. When the virus adapts to this temperature and can cause more than 80% of the cells to become diseased in about 48 hours, lower the temperature of the incubator by 1-2°C, and so on, and gradua...
Embodiment 2
[0032] Example 2, Safety Evaluation of Porcine Pseudorabies Virus TP Strain (PRV TP, CGMCC No.12300) to Susceptible Animals
[0033] 1) Pathogenicity test of porcine pseudorabies virus TP strain to Balb / c mice
[0034] 55 Balb / c mice aged 7-8 weeks, 5 in each cage. The PRV TP strain and rPRV-gE - -EGFP + Virus strain (microorganism preservation number is CGMCC NO.6657) was diluted to 10 2.0 -10 6.0 TCID 50 / 0.2mL, 5 mice (1 cage) were inoculated with each dilution of the virus, each with 0.2mL. The other 5 mice served as negative controls. Observe and record the death situation of mice every day after inoculation, the results are shown in Table 1, calculate the half lethal dose LD of two strains of viruses to mice 50 . The results showed that the median lethal dose (LD) of PRV TP strains to mice 50 ) is 10 5.17 TCID 50 , while rPRV-gE - -EGFP + LD in mice 50 for 10 3.32 TCID 50 .
[0035] Table 1 Comparison of pathogenicity of different strains to Balb / c mice ...
Embodiment 3
[0042] Embodiment 3, the immune protection efficacy test of porcine pseudorabies virus TP strain to piglets
[0043] Fifteen 21-day-old piglets that were negative for PRV by antibody detection were randomly divided into 3 groups with 5 piglets in each group. Group A each intramuscularly injects 1 head portion (every head portion virus content is 10 5.0 TCID 50 ) PRV Bartha K61 vaccine, each head of group B was intramuscularly injected with 1 head (every head virus content was 10 5.0 TCID 50 ) PRV TP strain, group C was used as the challenge control. 14 days after immunization, each pig was inoculated with 2 mL containing 10 6.0 TCID 50 F5 generation of PRV HeN1 strain. Blood was collected weekly after immunization to separate serum, and anti-PRV gB and gE antibodies were detected using IDEXX kits. Body temperature was measured daily for 14 days after challenge, and clinical responses were observed. The results are shown in Table 3. The results showed that most of the pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com