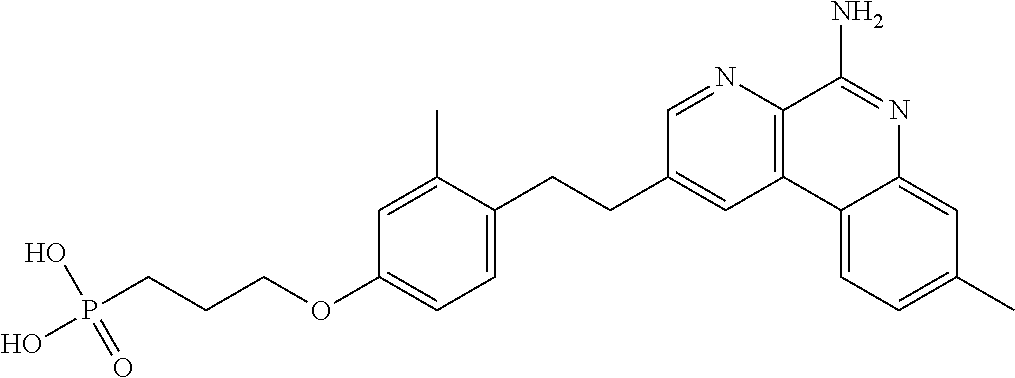
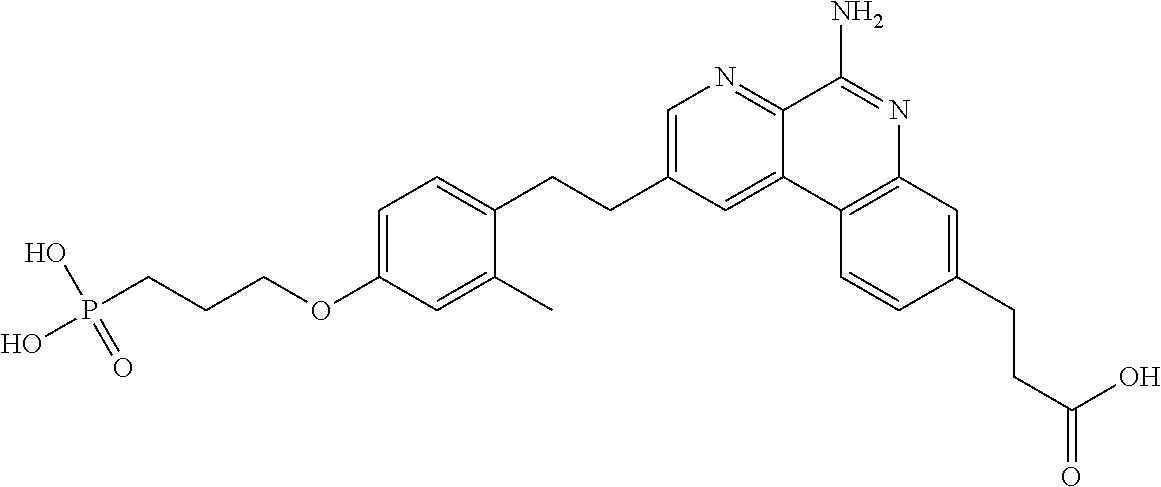
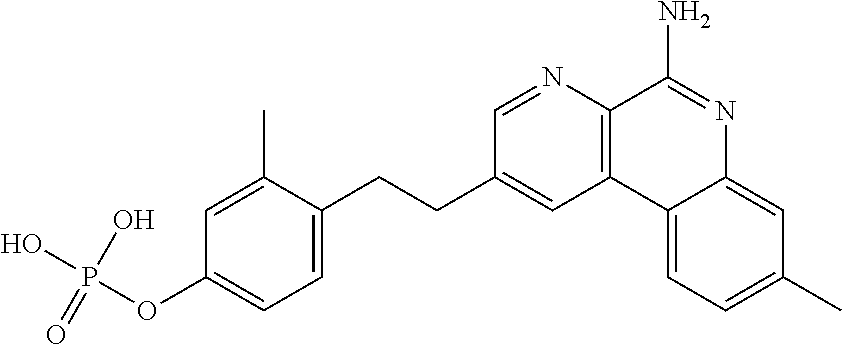
Combination vaccines with lower doses of antigen and/or adjuvant
a vaccine and vaccine technology, applied in the field of conjugated vaccines, can solve the problem of requiring relatively high amounts of antigen, and achieve the effect of fewer doses and without loss of immunoprotective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Adjuvant Adsorption to Antigens
[0196]3-valent (DTaP) or 6-valent (DTaP-HBsAg-IPV-Hib) vaccines were adjuvanted with aluminium hydroxide alone, aluminium hydroxide with pre-adsorbed ‘compound T’, poly(lactide-co-glycolide) microparticles (‘PLG’), and MF59 oil-in-water emulsion. Aluminium hydroxide and aluminium hydroxide with pre-adsorbed ‘compound T’ were prepared in histidine buffer pH 6.5. At pH 6.5, aluminium hydroxide has a positive net charge, while most proteins have a negative net charge. The pH value was chosen to provide good adsorption of most of the tested antigens. All formulations adjuvanted with aluminium hydroxide or aluminium hydroxide with pre-adsorbed ‘compound T’ showed optimal pH (6.5-6.8±0.1) and osmolarity values (0.300±50 mO). Osmolarity was adjusted with NaCl. Antigens for the MF59-adjuvanted formulations were prepared in PBS. The resulting preparations had pH values between 6.2 and 7.3 and osmolarity values around 0.300±50 mO. Formulations containing PLG mic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Force | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com