Kit for detecting circulating antigen indirect hemagglutination of schistosomiasis and manufacturing method thereof
A technology of schistosomiasis and kits, applied in the field of bioengineering, can solve problems such as inability to distinguish past infection from current infection, low sensitivity of circulating antigens, and inability to conduct curative effect assessment, achieving considerable economic and social benefits, easy promotion, high The effect of practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Anti-Schistosome japonicum egg antigen (SEA) specific IgY preparation and purification
[0027] 1. Preparation of soluble antigen of Schistosoma japonicum: 7 New Zealand rabbits each infected with 1500 cercariae of Schistosoma japonicum (from China Center for Disease Control and Prevention, Parasitic Disease Prevention and Control Institute) were dissected according to the conventional method after 42 days, and separated and collected from the liver eggs and freeze-dried. Take the dry eggs and weigh them, dissolve them in 0.9% normal saline at a ratio of 1%, place them in a cold soak at 4°C for 4 days, and shake them twice a day for 2 minutes each time. After 4 days, ultrasonic pulverization was performed in an ice bath, 3 minutes each time, with a 3-minute interval, 3 times in total. Then cold soak for 2 days as above, centrifuge at 10,000 rpm at 4°C for 30 minutes, take the supernatant, collect and aliquot, measure the concentration of antigenic protein...
Embodiment 2
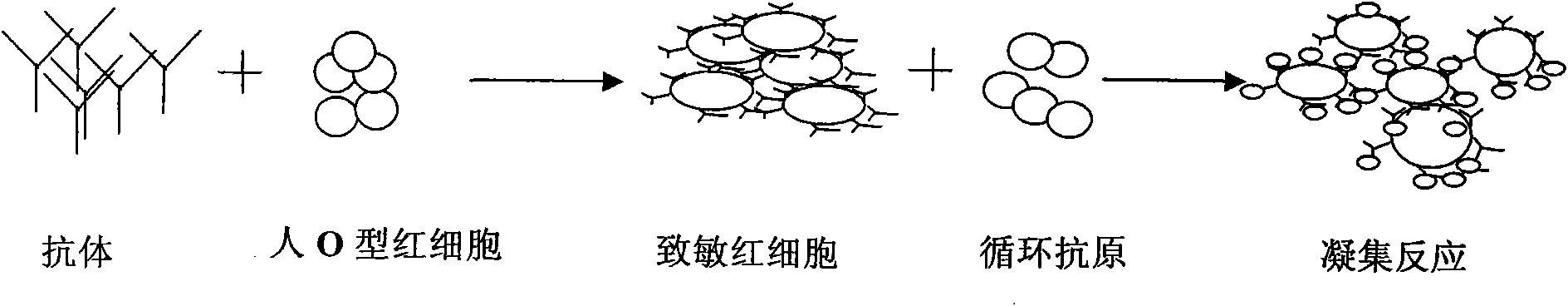
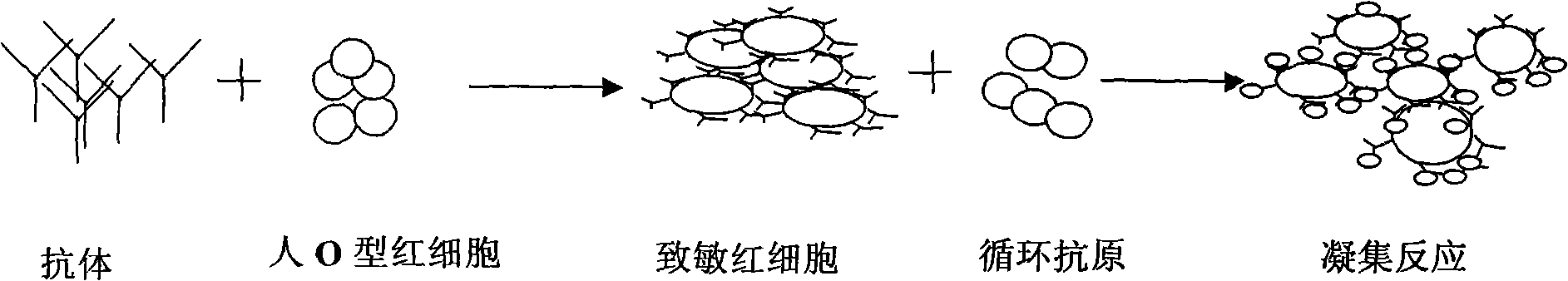
[0034] Embodiment 2: The present invention provides a kit for detecting schistosome circulating antigen indirect hemagglutination and its manufacturing method, including freeze-dried anti-SEA-IgY sensitized red blood cells, sensitized red blood cell diluent, specimen diluent, positive control substance, negative As for the control substance, the positive control substance is a standard dilution of Schistosoma japonicum soluble antigen, and the negative control substance is human normal serum.
[0035] Specifically, the sensitized erythrocyte diluent uses double distilled water (DDH 2 O), described sample diluent is 0.9% physiological saline.
[0036] Preparation of positive control standard product: Select 2.5GK healthy rabbits, infect each rabbit with 1500-2000 cercariae according to the conventional method, separate and collect eggs from the liver after 8 weeks and freeze-dry them. Take the dry eggs and weigh them, dissolve them in 0.9% normal saline at a ratio of 1%, place...
Embodiment 3
[0043] Example 3: Detection of Schistosoma Circulating Antigens Indirect Hemagglutination Kit Use:
[0044] 1. Operation steps:
[0045] (1) Prepare sensitized erythrocyte suspension: take freeze-dried sensitized erythrocytes, add 1ml of diluent, and mix well.
[0046] (2) Negative and positive controls should be set up for each test. Positive and negative control sera are lyophilized products, diluted with 100 μl distilled water in each tube before use, and used after fully dissolved.
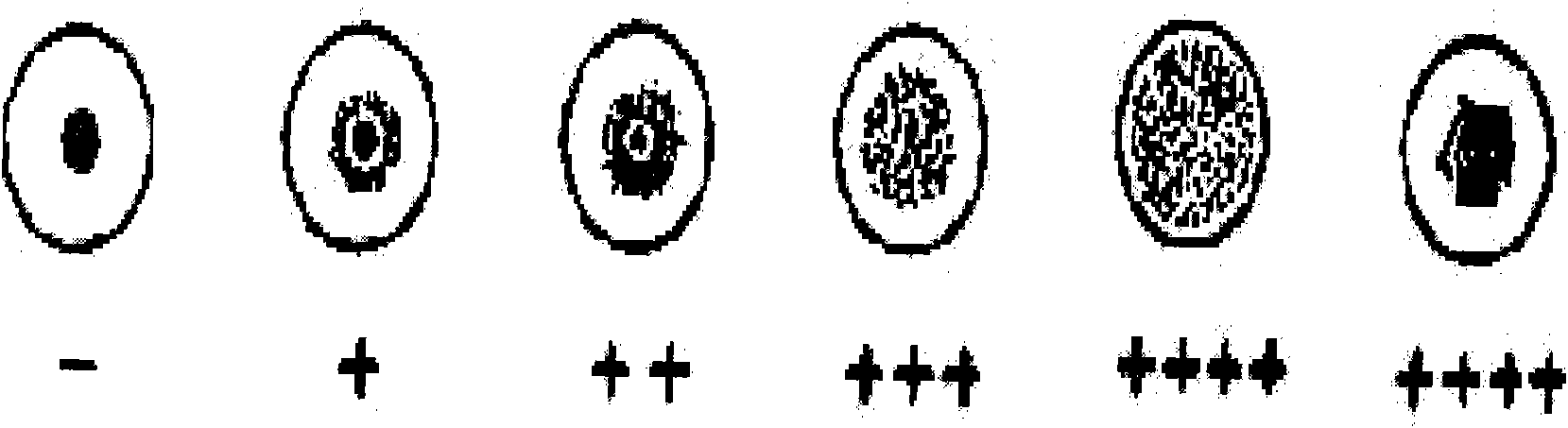
[0047] (3) Add 100 μl of sample diluent to the first well of the first row of the hemagglutination plate, and add sample diluent to the second to fourth wells. Add 30μl of the serum to be tested in the first well, mix well and suck out 30ul into the second well, mix well in the second well and then serially dilute to the fourth well, discard the excess 30μl after mixing in the fourth well, Serum dilutions from the second to fourth wells were 1:10, 1:20 and 1:40, respectively. Then add 1 dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| sedimentation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com