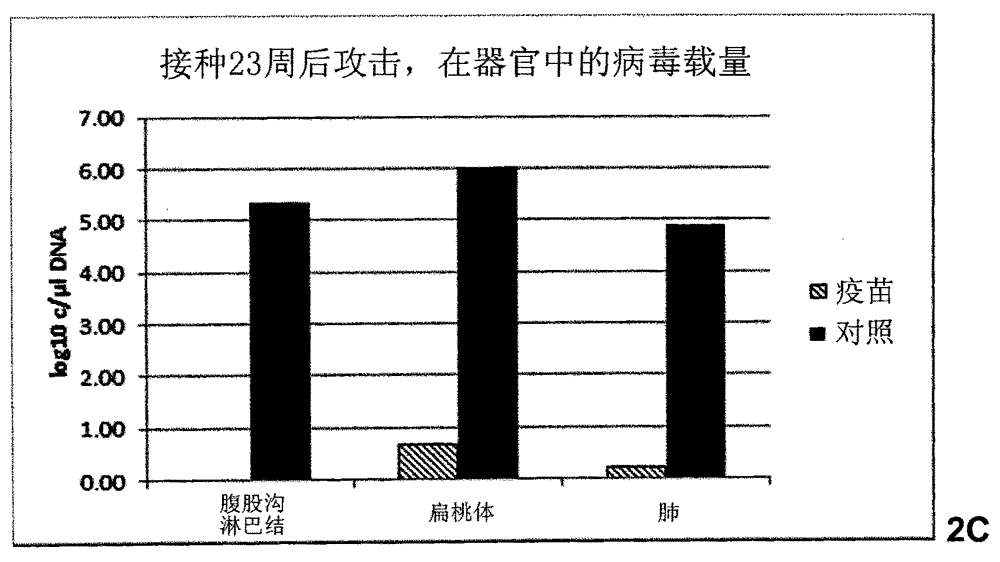
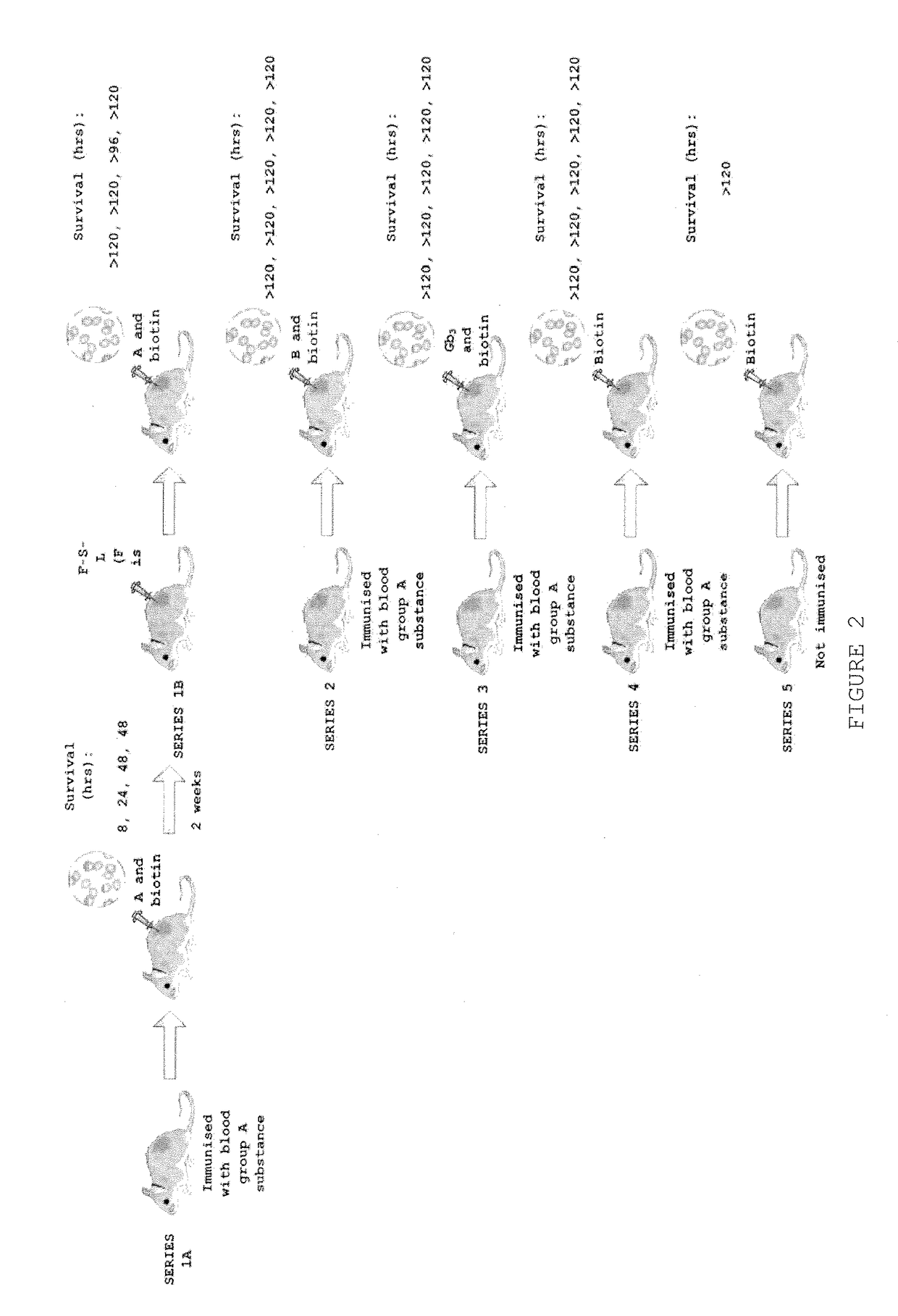
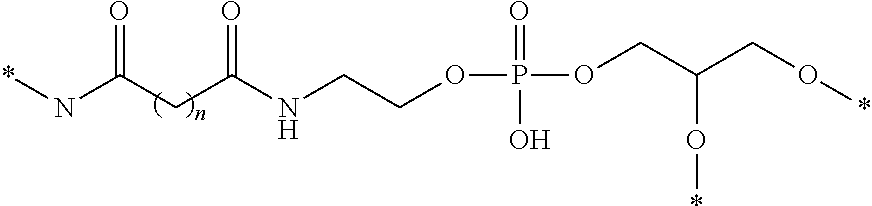
Patents
Literature
32 results about "Circulating antibodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
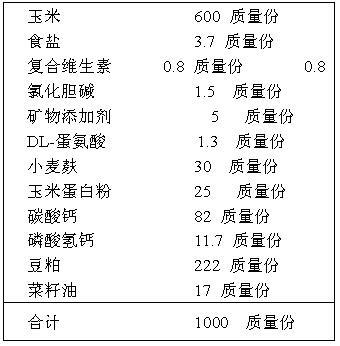
A form of immunity mediated by circulating antibodies (immunoglobulins IgA, IgB, and IgM), which coat the antigens and target them for destruction by polymorphonuclear neutrophils. Circulating antibodies are produced by plasma cells of the reticuloendothelial system.
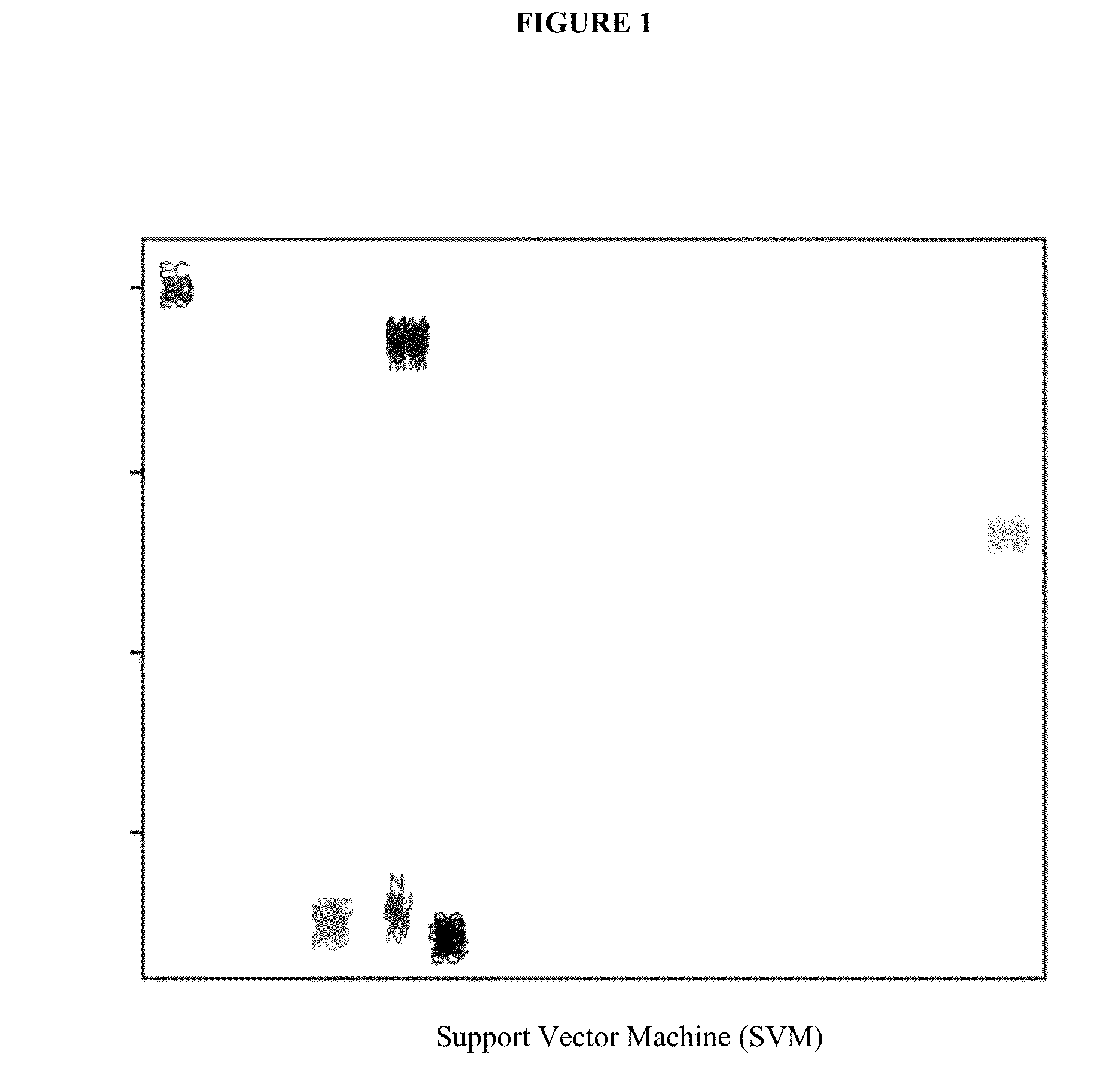
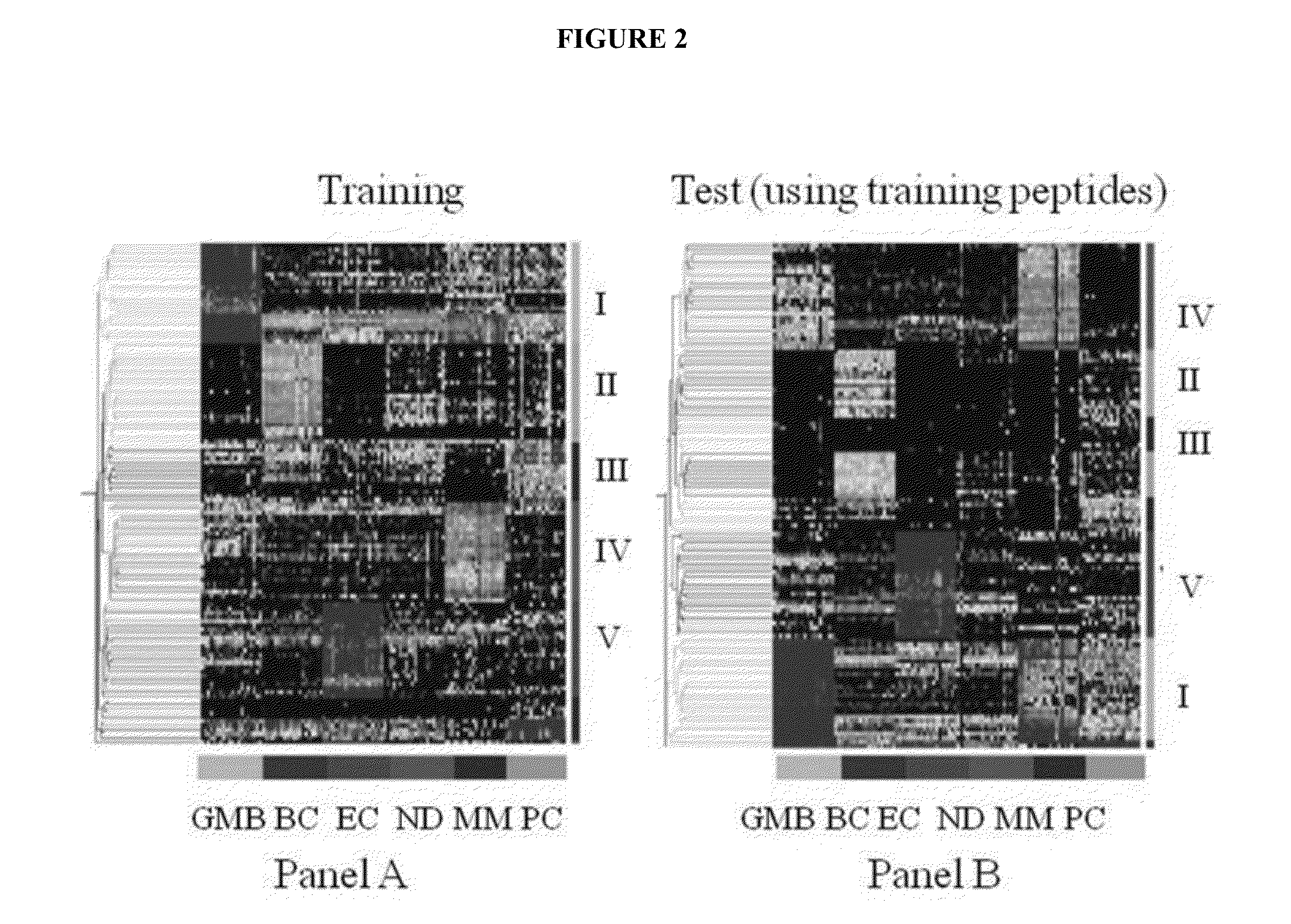
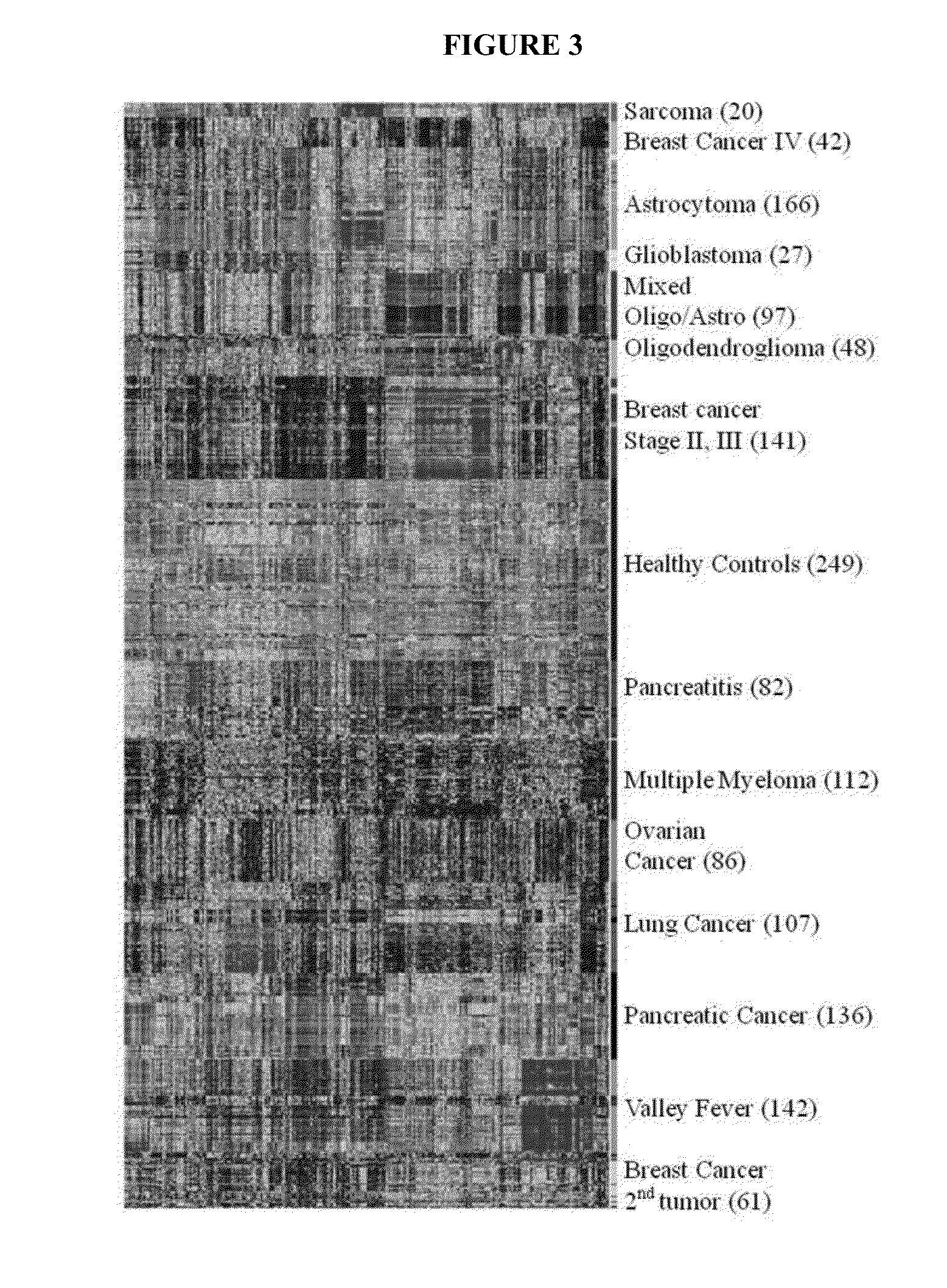
Immunosignaturing: a path to early diagnosis and health monitoring
InactiveUS20140087963A1Robust methodReduce in quantityMaterial nanotechnologyPeptide librariesDiagnosis earlyState of health
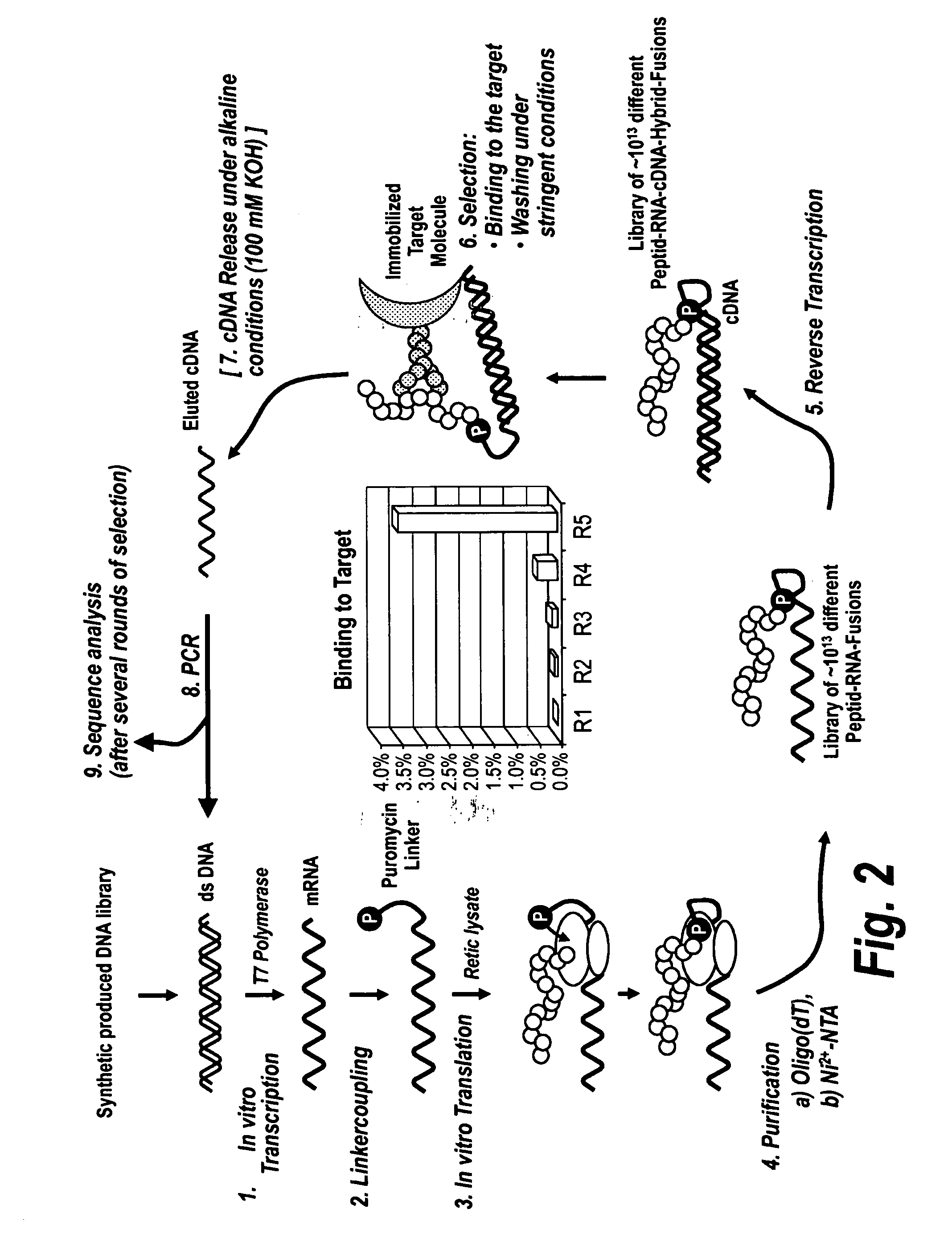
Health is a complex state that represents the continuously changing outcome of nearly all human activities and interactions. The invention provides efficient methods and arrays for health monitoring, diagnosis, treatment, and preventive care. The invention monitors a broad range of identifying molecules from a subject, such as circulating antibodies, and the invention evaluates a pattern of binding of those molecules to a peptide array. The characterization of the pattern of binding of such molecules to a peptide array with the methods of the invention provide a robust measure of a state of health of a subject.
Owner:ARIZONA STATE UNIVERSITY
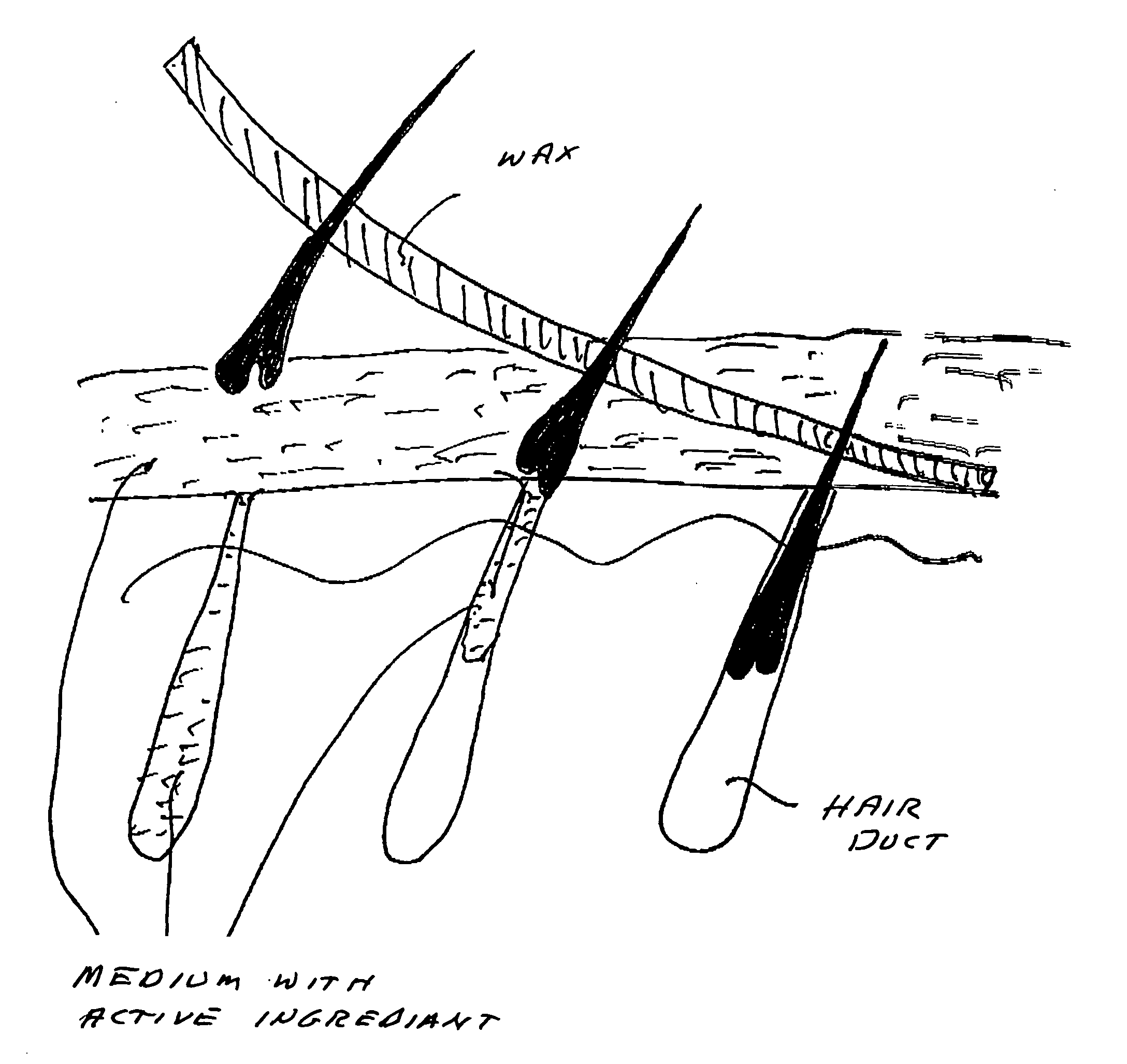
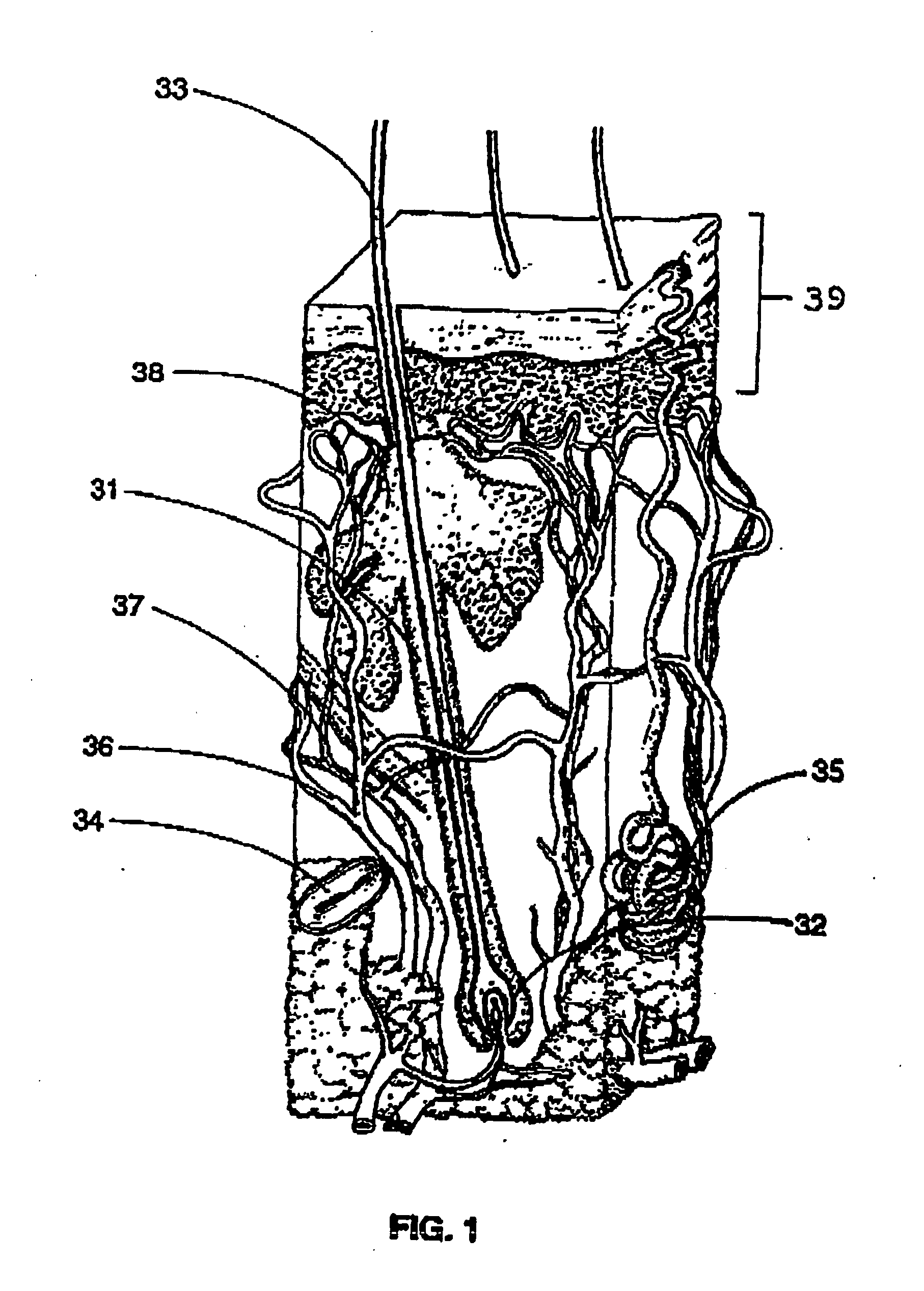
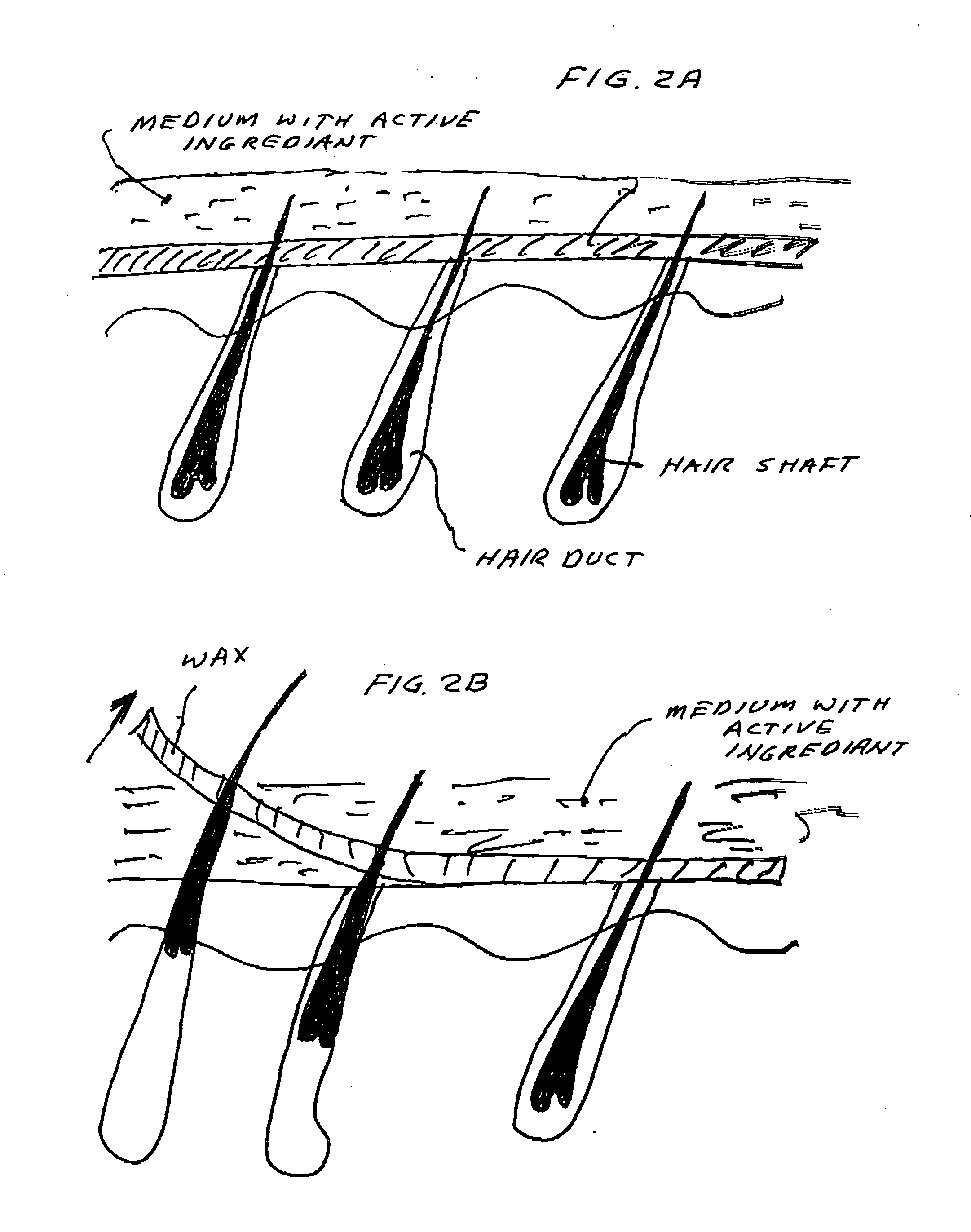
Vaccine for hair removal
A process for delivering hair removal fluids to hair ducts. In a preferred embodiment the fluid is a vaccine for slowing or stopping hair growth and a process for applying the vaccine or other hair growth vaccines to the skin region having unwanted hair and a method of delivering the vaccine to skin regions of unwanted hair. The preferred vaccine consists of an antigen cocktail of weakened or essentially dead elements of hair follicles for stimulating antibody responses. The preferred vaccine is prepared from patient's own hair papilla or bulge area stem cells. In other embodiments vaccines are made from extracts of papilla cells cultivated in a laboratory. The vaccines produce antibodies and these antibodies attack and destroy hair papilla and block bulge area cells that would otherwise produce or stimulate a growth of new papilla cells. Even if any new papilla cells appear, the circulating antibodies of the vaccine-activated immune system attack and destroy them also. Other hair removal fluids that can be applied with the process of this invention are also described.
Owner:TANKOVICH NIKOLAI
Therapeutic targeting of escort proteins
The invention provides for inhibition of viral disease by the provision to a mammalian host of antibodies directed against an escort protein likeTsg 101. These proteins appear on the surface of a cell, and thus can be bound by circulating antibodies thereto. By binding escort proteins on the cell surface, budding of viral particles is inhibited. The virus infects the initial cells, but cannot escape that cell to infect the body en masse.
Owner:ELI LILLY & CO
Immunological modulation of insulin-like growth factor 1 for cancer prevention/treatment and prolonging longevity
ActiveUS7749966B2Simple and safe and effectiveReduce morbidityAntibacterial agentsPeptide/protein ingredientsCancer preventionInsulin-like growth factor
Owner:RASO VICTOR DR
Methods and formulations for reducing circulating antibodies
InactiveUS20050220785A1Lower Level RequirementsOrganic active ingredientsMammal material medical ingredientsEpitopeDisease cause
The invention provides methods for reducing circulating levels of antibodies, particularly disease-associated antibodies. The methods entail administering effective amounts of epitope-presenting carriers to an individual. In other embodiments, ex vivo methods for reducing circulating levels of antibodies are provided which employ epitope-presenting carriers.
Owner:ENGLE STEVEN B +3
Modified antibodies with human milk fat globule specificity and uses
InactiveUS6936706B2Reduce serum concentrationReduce concentrationAnimal cellsIn-vivo radioactive preparationsVaccinationNucleotide
An analogue peptide that comprises the variable regions of the light or heavy chains of an antibody of a first species selectively binding to a carcinoma antigen has 1 to 46 amino acids of the framework regions per chain substituted with amino acids such as those present in equivalent positions in antibodies of a species other than the first species, or fragments thereof comprising 1 to 3 variable region CDRs per chain and optionally flanking regions thereof of 1 to 10 or more amino acids, alone or with an N-terminal fragment of 1 to 10 or more amino acids, combinations or mixtures thereof. The polypeptide may also comprise an effector agent and / or be glycosylated, and is presented as a composition with a carrier. The analogue peptides are used in diagnostic kits for carcinomas and methods for in vivo imaging and treating a primary or metastasized carcinoma, and in vitro diagnosing a carcinoma, ex vivo purging neoplastic cells from a biological fluid. RNAs and DNAs encode the analogue peptide, and a hybrid vector carrying the nucleotides and transfected cells express the peptides and a method produces the analogue peptide. An anti-idiotype polypeptide comprises polyclonal antibodies raised against an anti-carcinoma antibody or the analogue peptide of this invention, monoclonal antibodies thereof, Fab, Fab′, (Fab′)2, CDR, variable region, or analogues or fragments thereof, combinations thereof with an oligopeptide comprising a TRP trimer, tandem repeats thereof, or combination or mixtures thereof. An anti-idiotype hybrid polypeptide with an effector agent and the anti-idiotype polypeptide, an anti-carcinoma vaccine, an anti-carcinoma vaccination kit, a method of vaccinating against carcinoma and a method of lowering the serum concentration of a circulating antibody or polypeptide are provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
A kind of production method of egg with high immunoglobulin content
The invention provides a novel technique and a novel method for producing novel health care food, i.e. a technique and a method for producing health care eggs with high immune globulin content by biological nanometer materials. The invention is characterized in that compound feed which supplies nutrition is designed and prepared according to the nutrition physiology characteristics of the immune system of a laying hen to be used as base effect substance, organic atomic state nano selenoprotein is used as a main effect nutrition factor by the special biology effect, iodine from feed-grade potassium iodide and escherichia coli vaccine immune are combined to be used as auxiliary effect factors for cooperating with the effect of nano-selenium so as to fully stimulate the function of the immune system of the laying hen, improve the circulating antibody level of the hen, increase the antibody deposition in an egg secretion process and form eggs with high immune globulin content. With the method and the technique, the immune globulin content of the egg can be increased by more than 36%. The contribution rate of the synergy of the invention to the immune globulin output of the egg can reach more than 62%.
Owner:福建省新闽科生物科技开发有限公司
In vitro assay for quantitating secreted antibodies in lymphocyte supernatant for evaluation of vaccine or antigen induced specific antibody secretion from ex vivo circulating antibody-secreting lymphocytes
The present invention provides methods of quantitating recent secreted antigen specific antibodies from supernatant of antibody secreting cells (ASC) in vitro culture for evaluation of vaccine or antigen induced antigen specific antibody secretion without ex vivo antigen stimulation.
Owner:CHANG HUI SUNNY
Therapeutic targeting of escort proteins
Owner:ELI LILLY & CO
Methods for blood typing and antibody screening
InactiveUS20170315141A1Prevent erythrocyte sedimentationHigh viscosityBiological testingAnalyteGroup A - blood
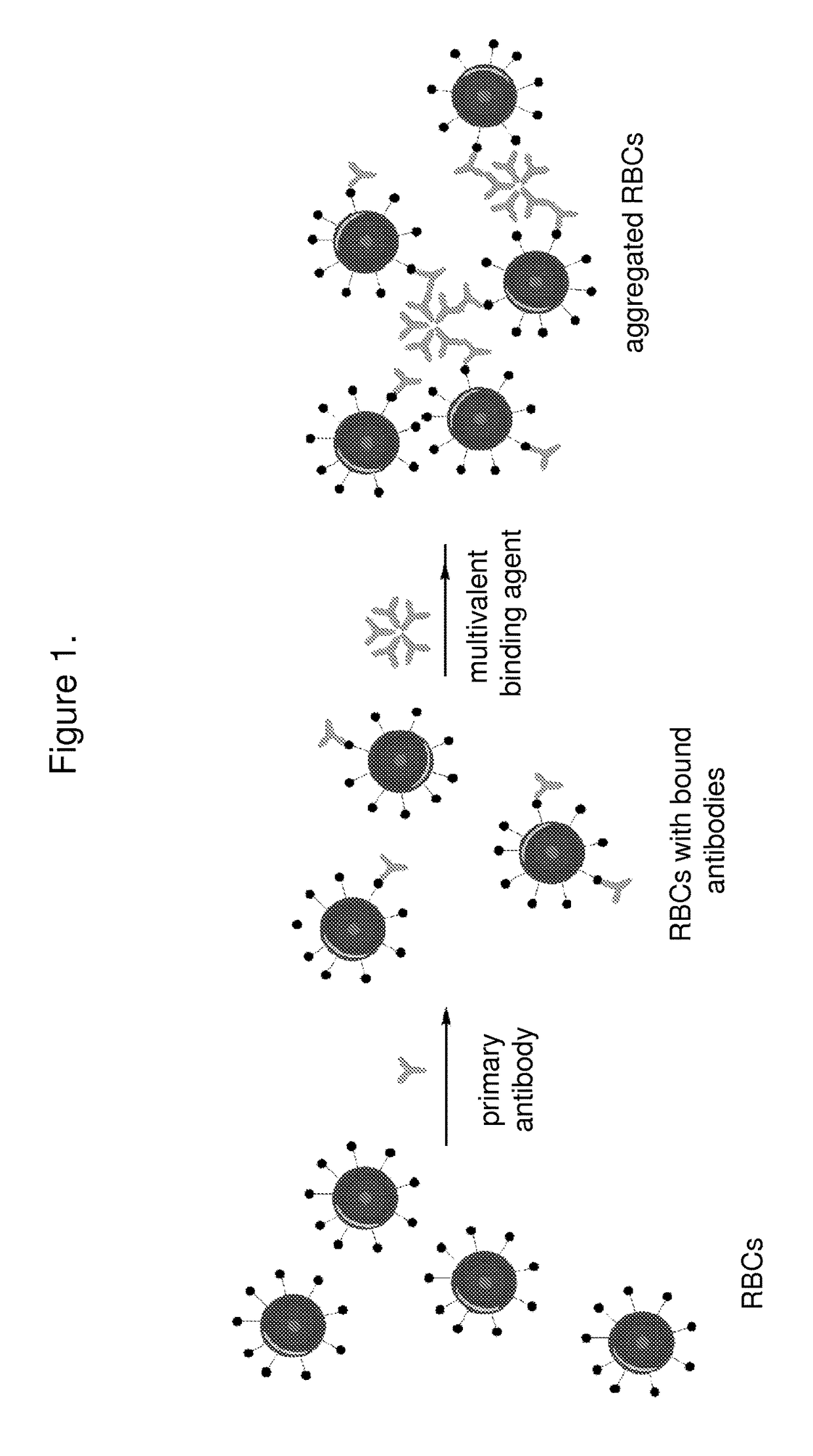
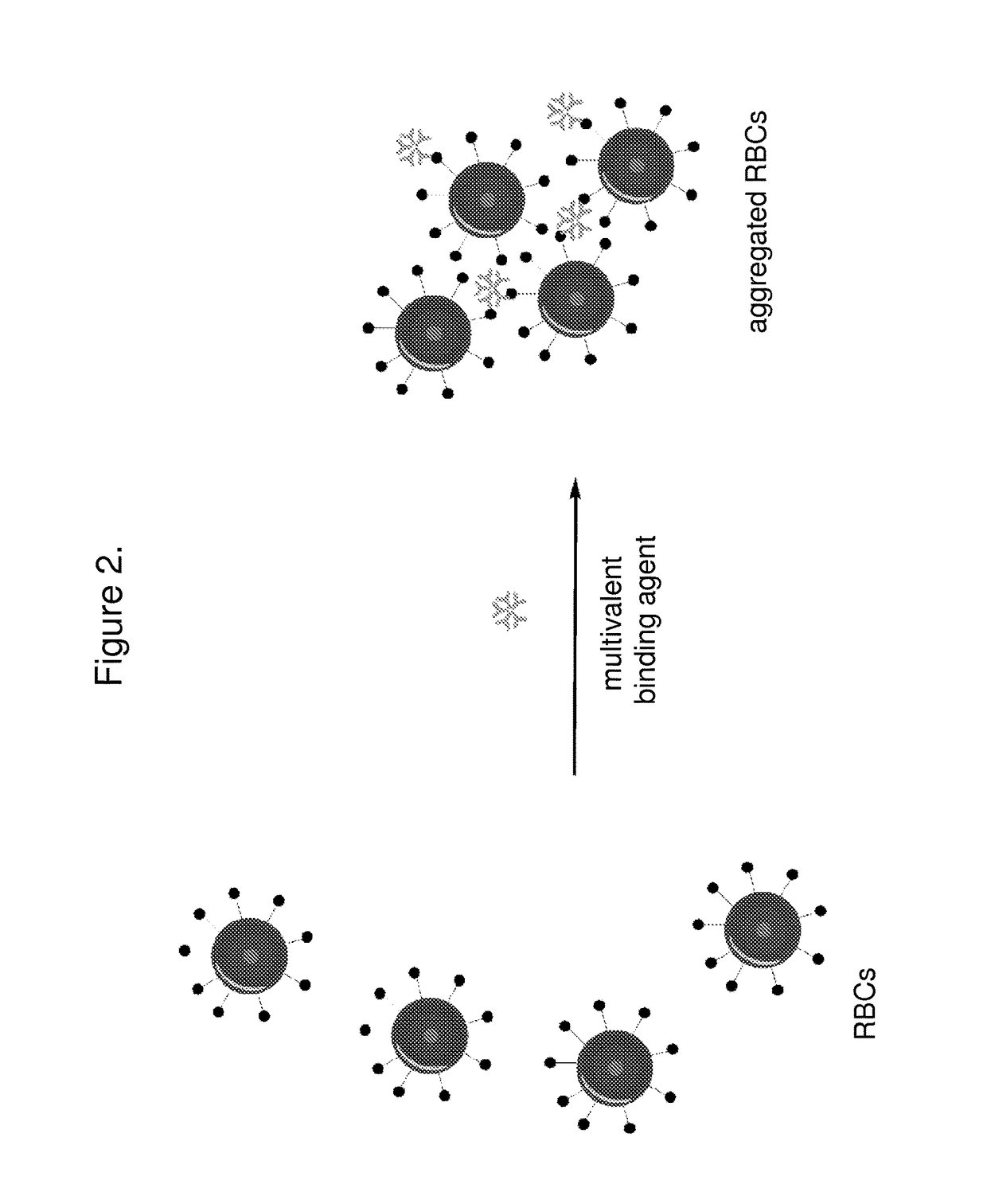
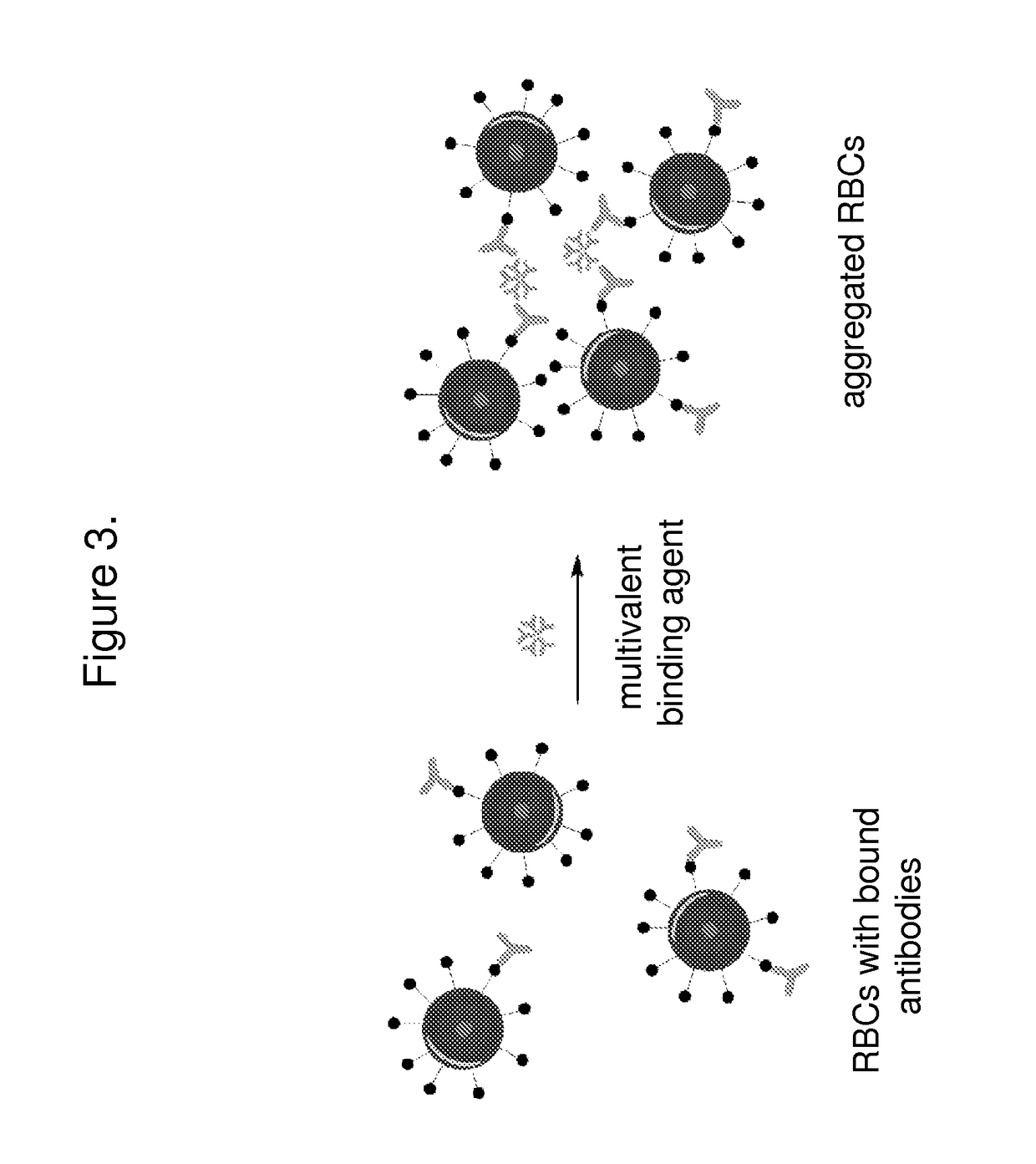
The invention features systems and methods for the detection of analytes, particularly antigens, complement, and antibodies present in blood. The invention features a system for the detection of antigens, complement, and antibodies on the surface of RBCs and circulating antibodies in the blood.
Owner:T2 BIOSYST
Application of fluorine-containing compound modified cationic polymer in preparation of vaccine drugs
ActiveCN111848831AEffectively induce the formation ofThe synthesis process is matureCosmetic preparationsOrganic active ingredientsPolymer modifiedBackbone chain
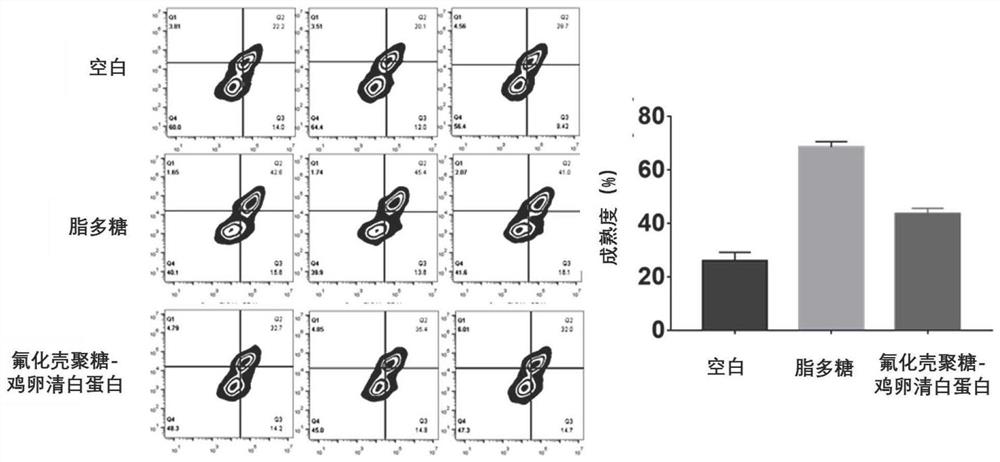
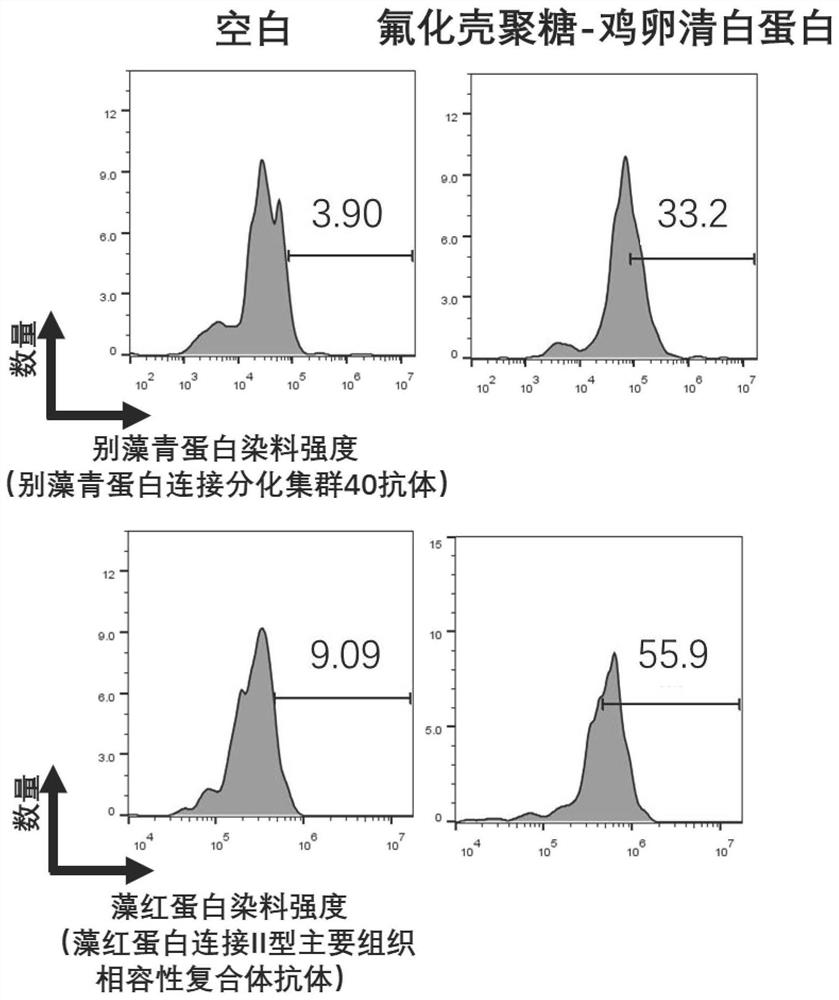
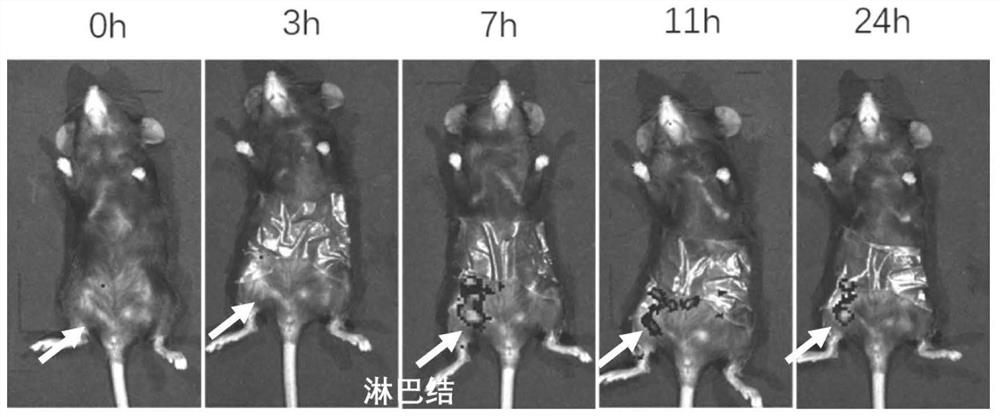
The invention discloses an application of fluorine-containing compound modified cationic polymer in preparation of vaccine drugs. The cationic polymer comprises a transdermal vaccine vector (a), wherein the transdermal vaccine vector (a) is a cationic polymer modified by a fluorine-containing compound; the cationic polymer modified by the fluorine-containing compound is fluorinated chitosan; a fluorine-containing compound is covalently connected to a main chain of chitosan, the molecular weight range of the chitosan is 1000-5000000, the deacetylation degree of the chitosan is not less than 55%, the viscosity range of the chitosan is 25-1000 centipoises, and the transdermal vaccine carrier has three antigen permeation paths including intracellular permeation, intercellular permeation and hair follicle permeation. Fluorinated chitosan is a very good immunologic adjuvant, can non-specifically stimulate an immune system, can effectively induce cytotoxic macrophages and cell-mediated immuneand circulating antibody formation, and amplifies immune response. The biocompatibility is good, and the huge commercial value is realized.
Owner:SUZHOU UNIV
Autoantibodies for protein antigens as markers for cancer of gingivo-buccal complex
ActiveUS20110189699A1Inhibit tumor cell growthFacilitate killingBiological testingAntibody medical ingredientsCellular antigensCancer cell
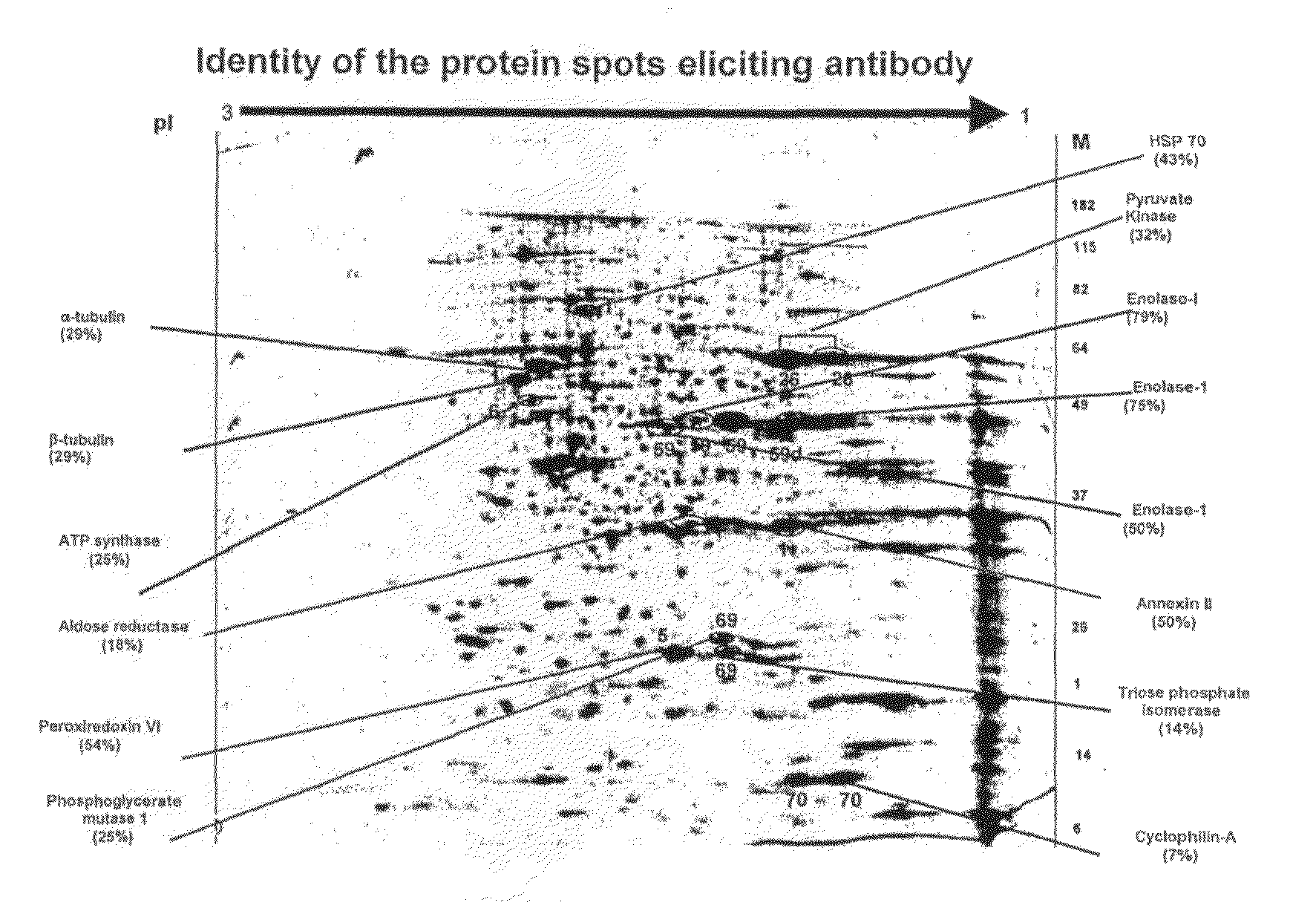
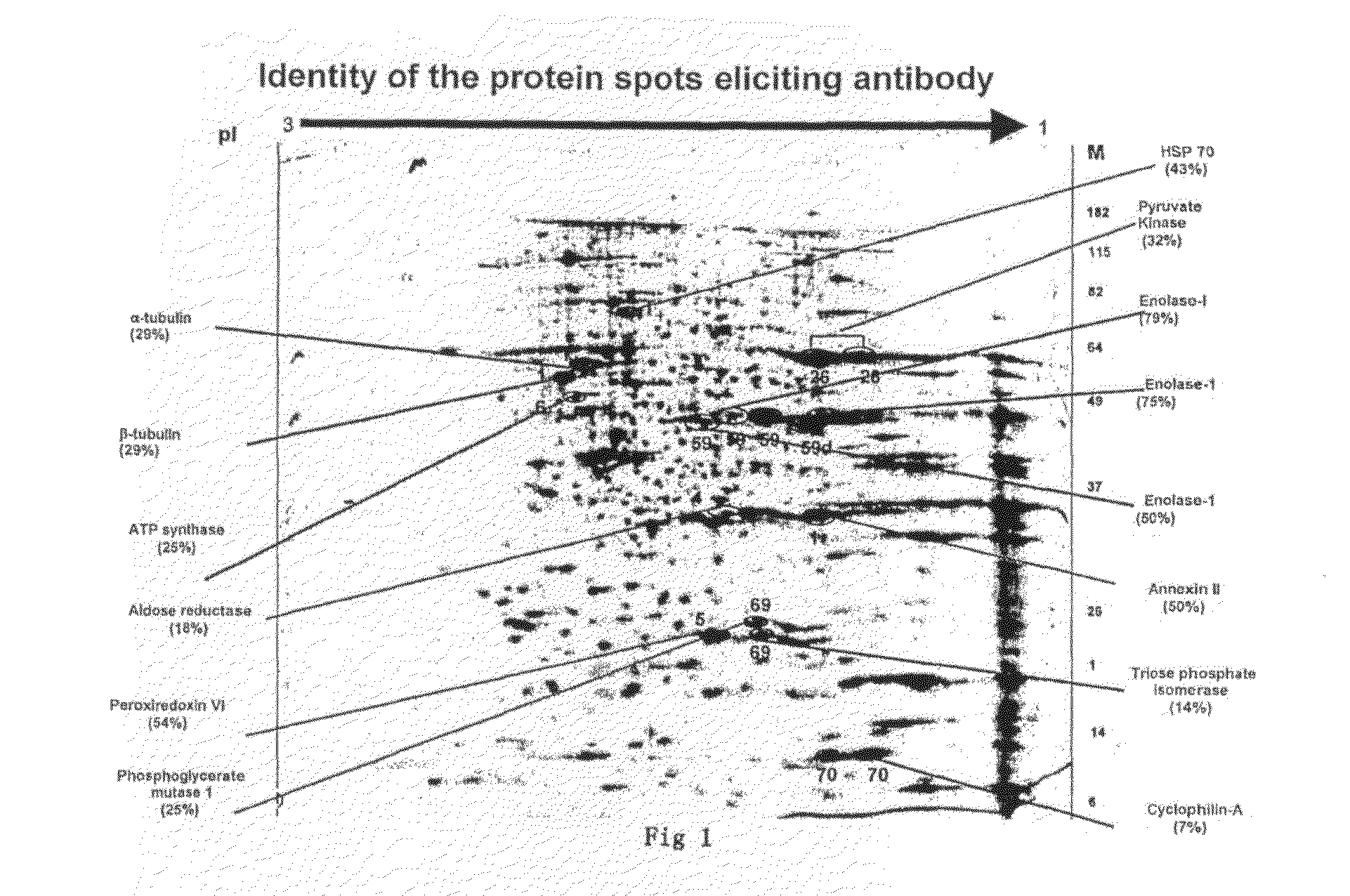
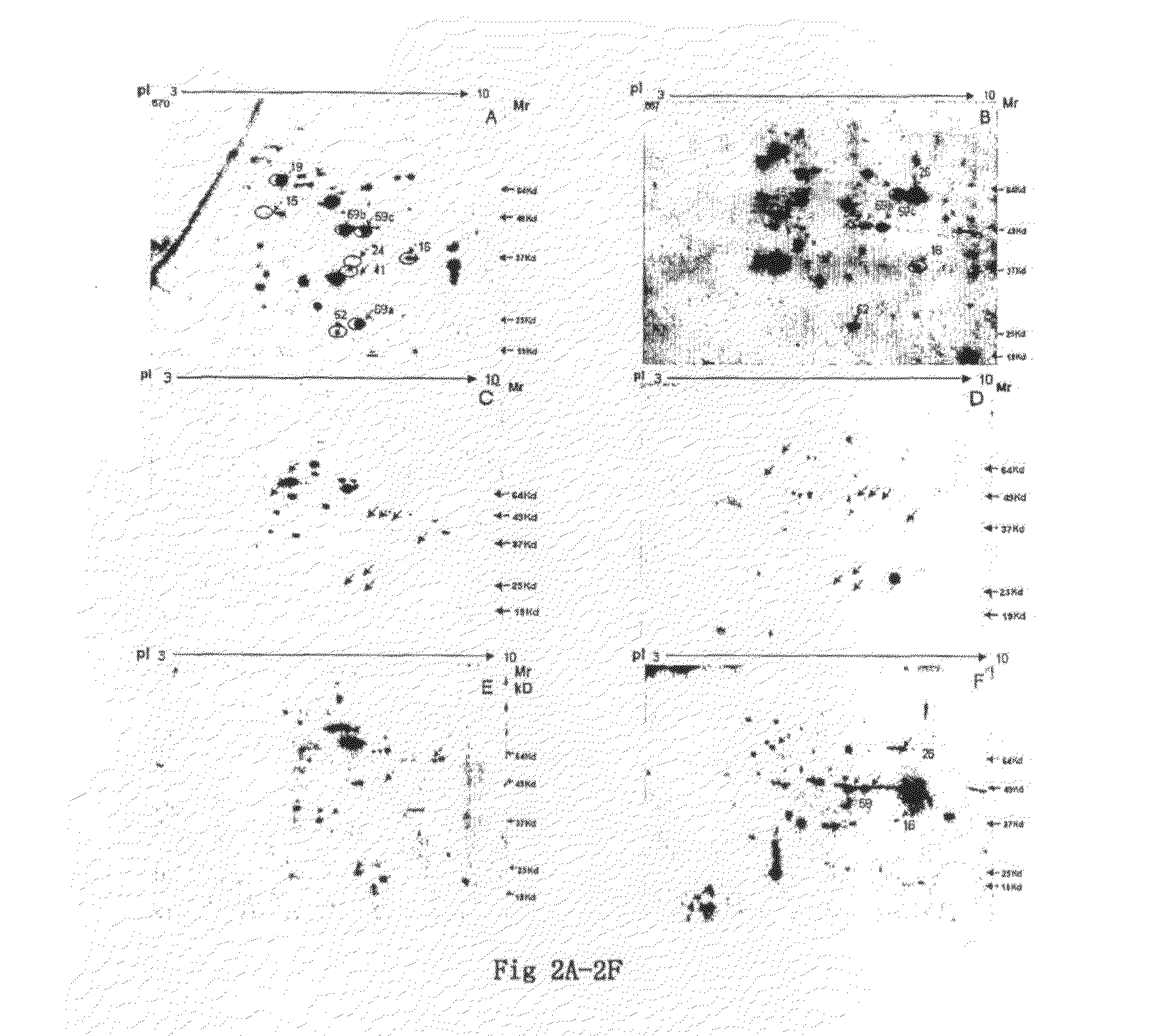
The present invention relates to identification of a set of proteins, which elicit an autoantibody response in patients with cancer of gingivo-buccal complex. Systematic comparisons of serum samples from clinically normal individuals and from patients with cancer of gingivo-buccal complex has revealed significant differences in the presence of autoantibodies in sera against cellular antigens present in a cancer cell line. The autoantibody response to a single or combination of these protein antigens serves as a novel marker and can be utilized for screening, early detection, prognosis, and potential target for therapy. The invention also provides for the use of the identified protein antigens in immunoassays designed to detect the presence of serum antibodies to the specific protein antigens in sera from individuals that harbor such antibodies. The invention also relates to the use of the identified antigens as immunogens for stimulation of an immune response in patients expressing such protein antigens. The invention is demonstrated by way of example in which elevated levels of circulating antibodies reactive against tumor specific antigens were identified in sera derived from patients with cancer of gingivo-buccal complex. The utility of identified antigens for early detection is assessed by analysis of sera from patients with leukoplakia of gingivo-buccal complex.
Owner:COUNCIL OF SCI & IND RES
Immunosignaturing: a path to early diagnosis and health monitoring
InactiveUS20200256861A1Reduce in quantityReduce stepsMaterial nanotechnologySequential/parallel process reactionsEmergency medicinePreventive healthcare
Health is a complex state that represents the continuously changing outcome of nearly all human activities and interactions. The invention provides efficient methods and arrays for health monitoring, diagnosis, treatment, and preventive care. The invention monitors a broad range of identifying molecules from a subject, such as circulating antibodies, and the invention evaluates a pattern of binding of those molecules to a peptide array. The characterization of the pattern of binding of such molecules to a peptide array with the methods of the invention provide a robust measure of a state of health of a subject.
Owner:ARIZONA STATE UNIVERSITY
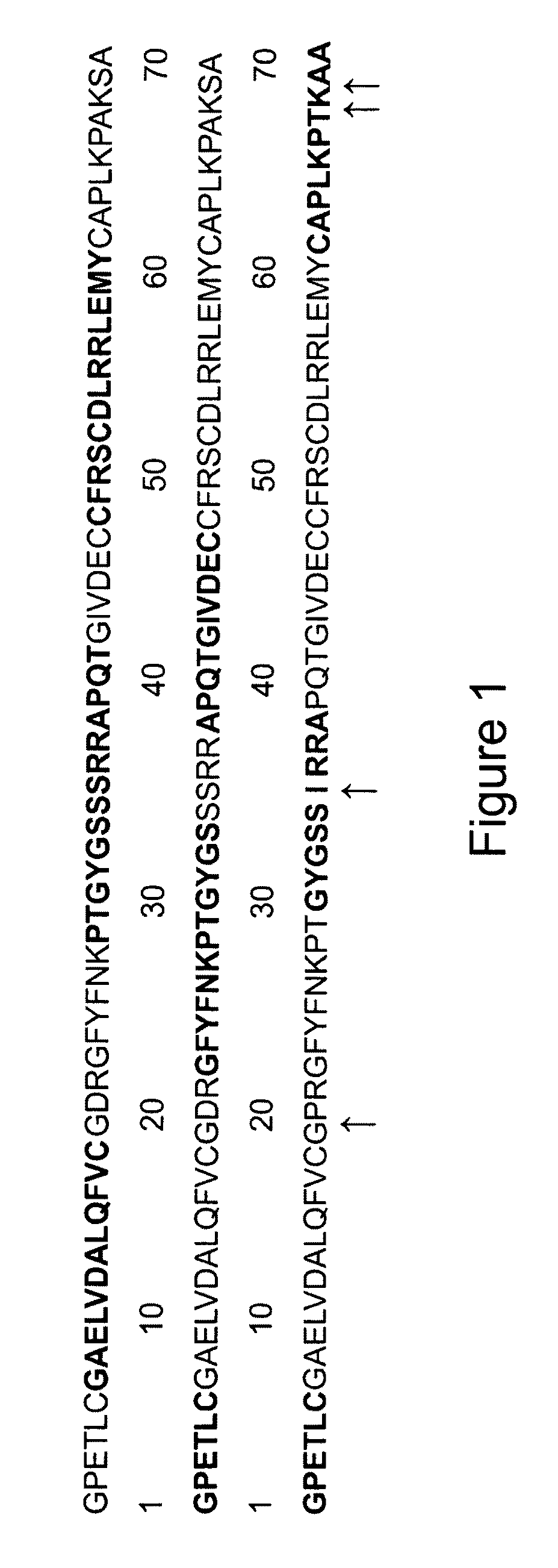
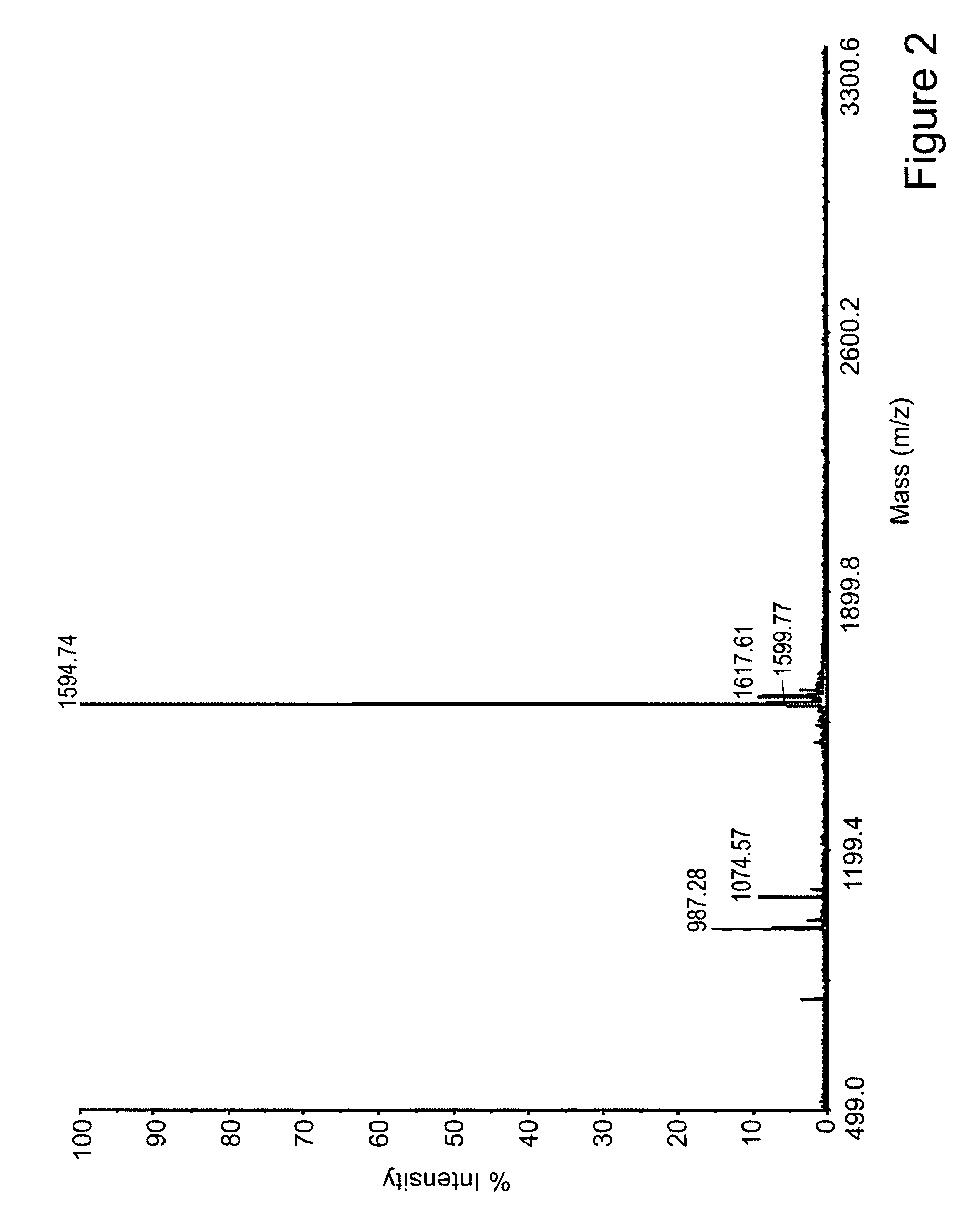
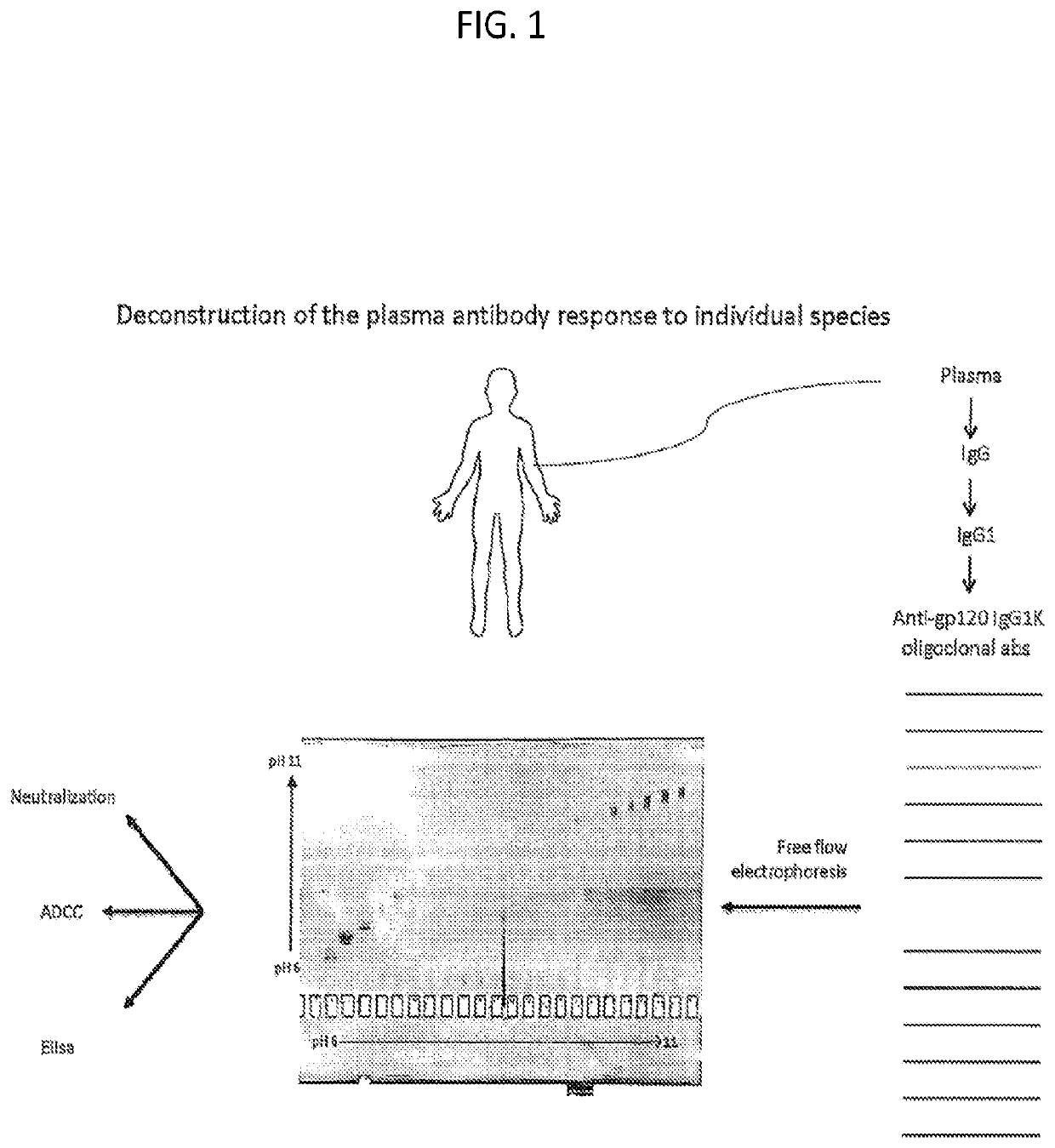
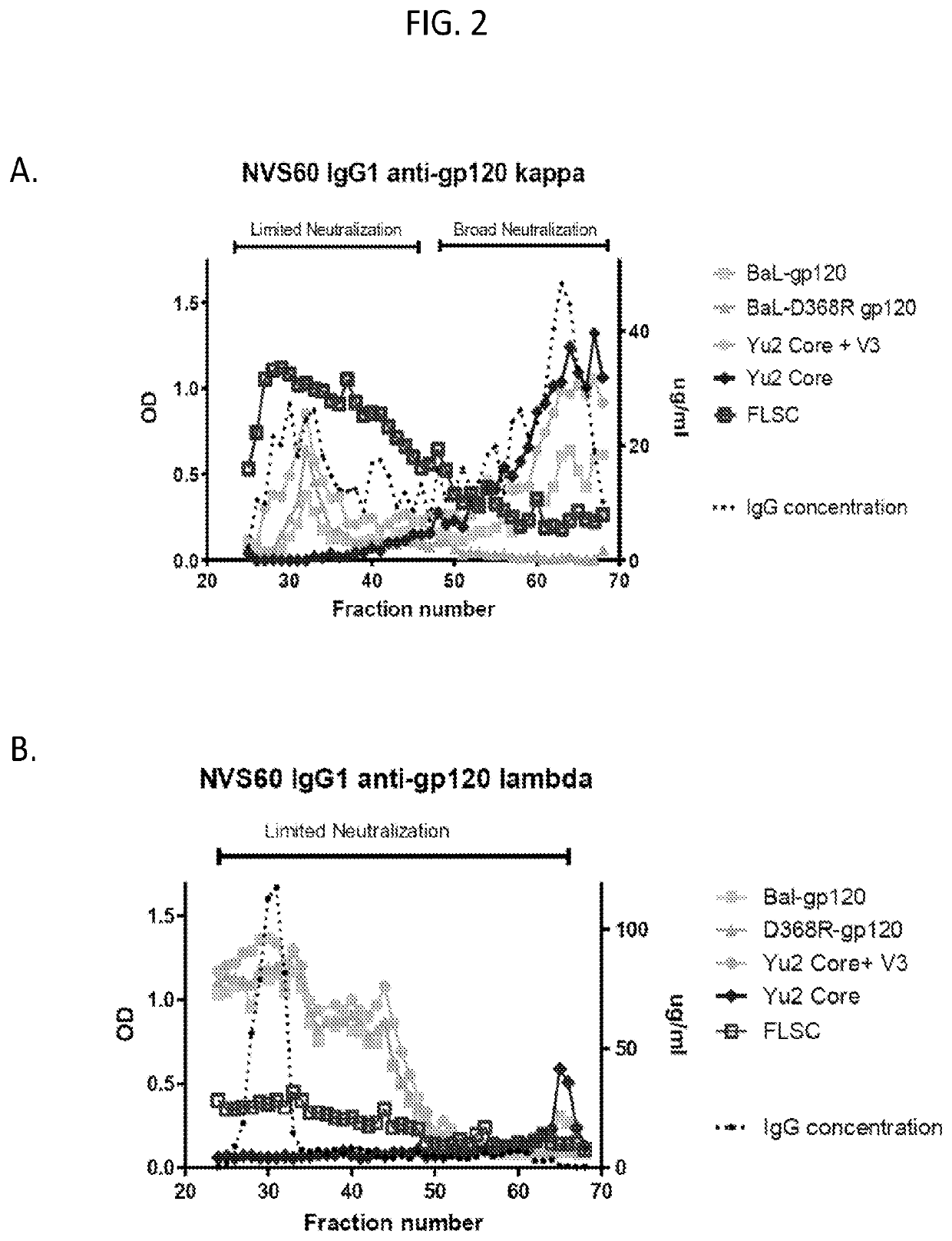
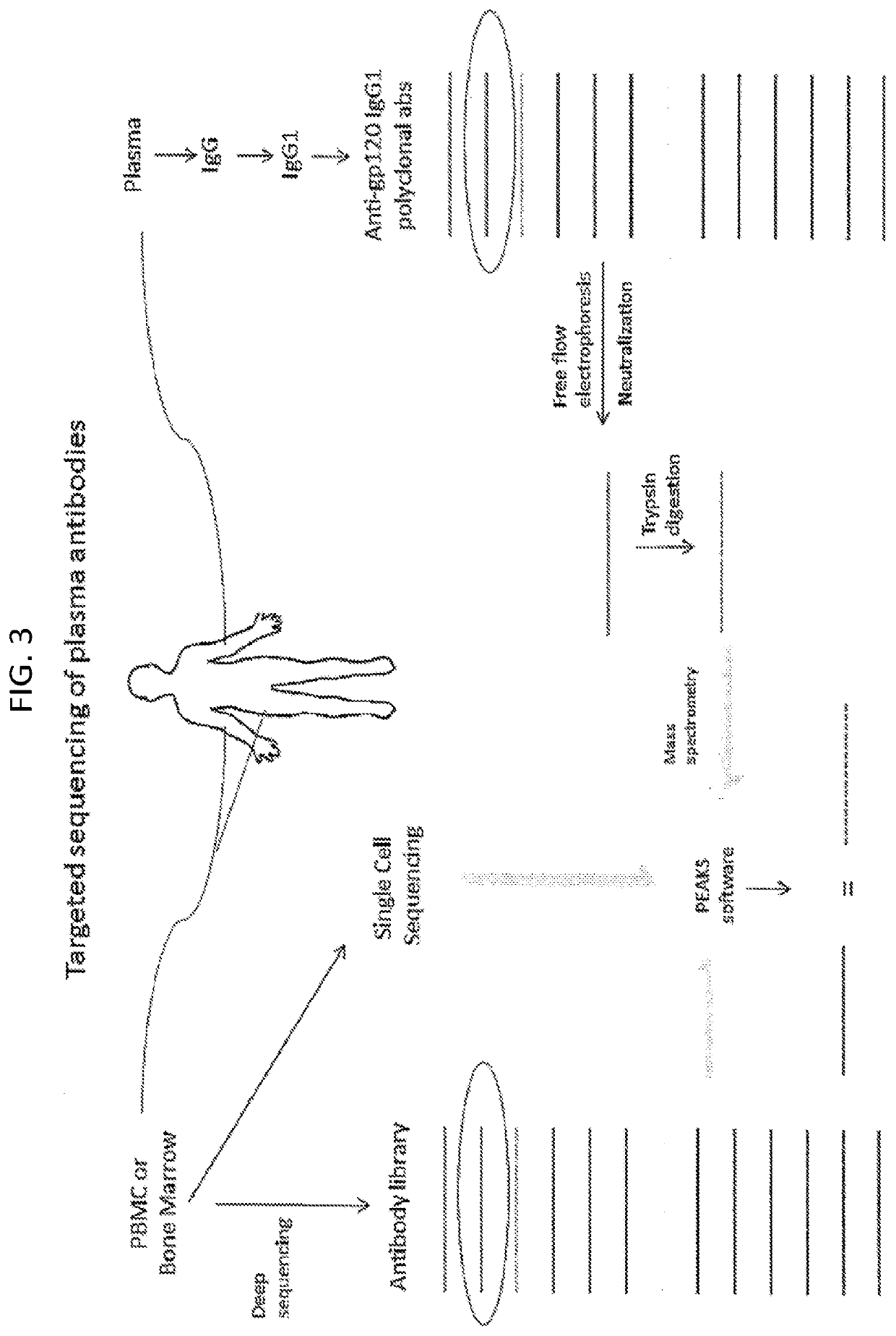
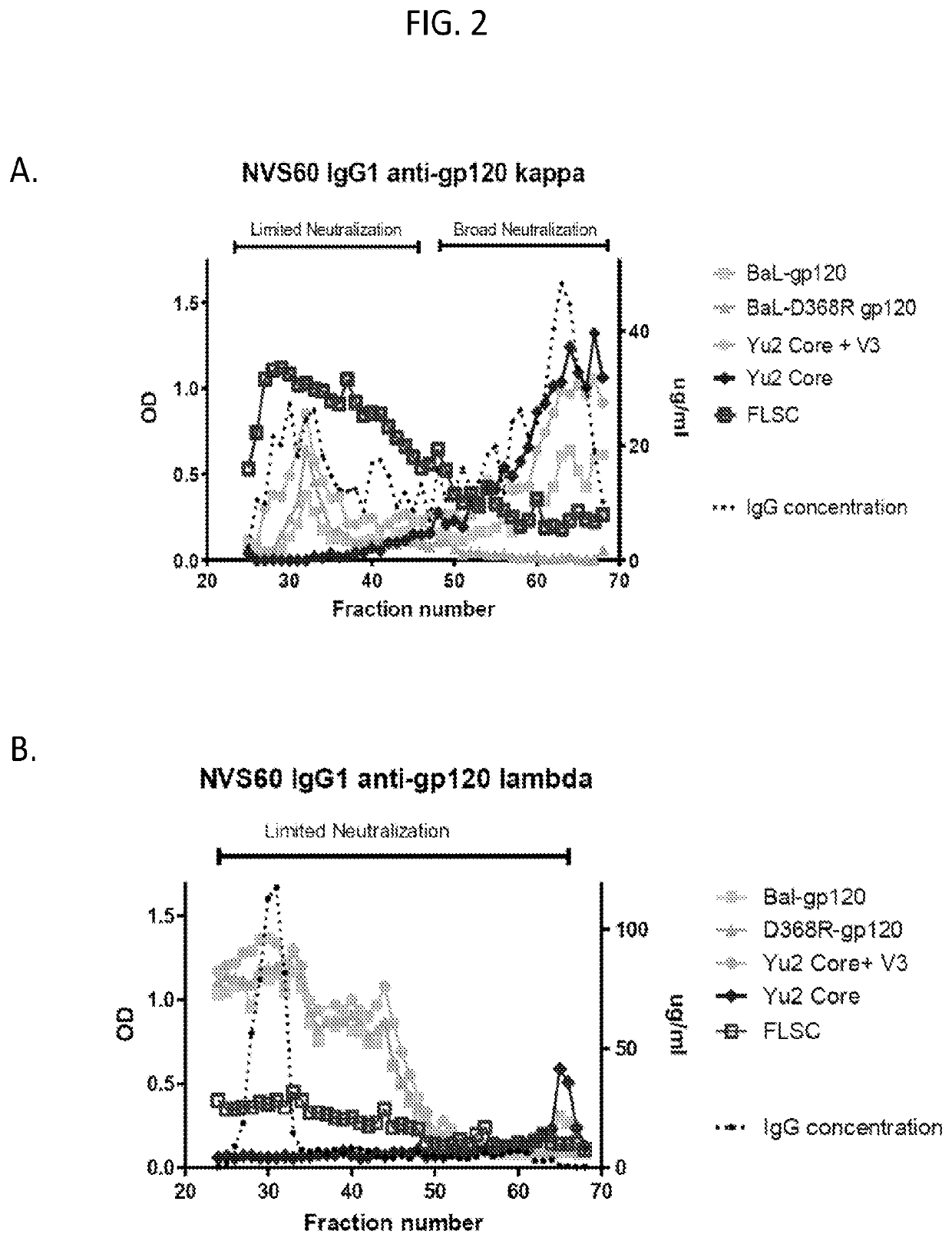
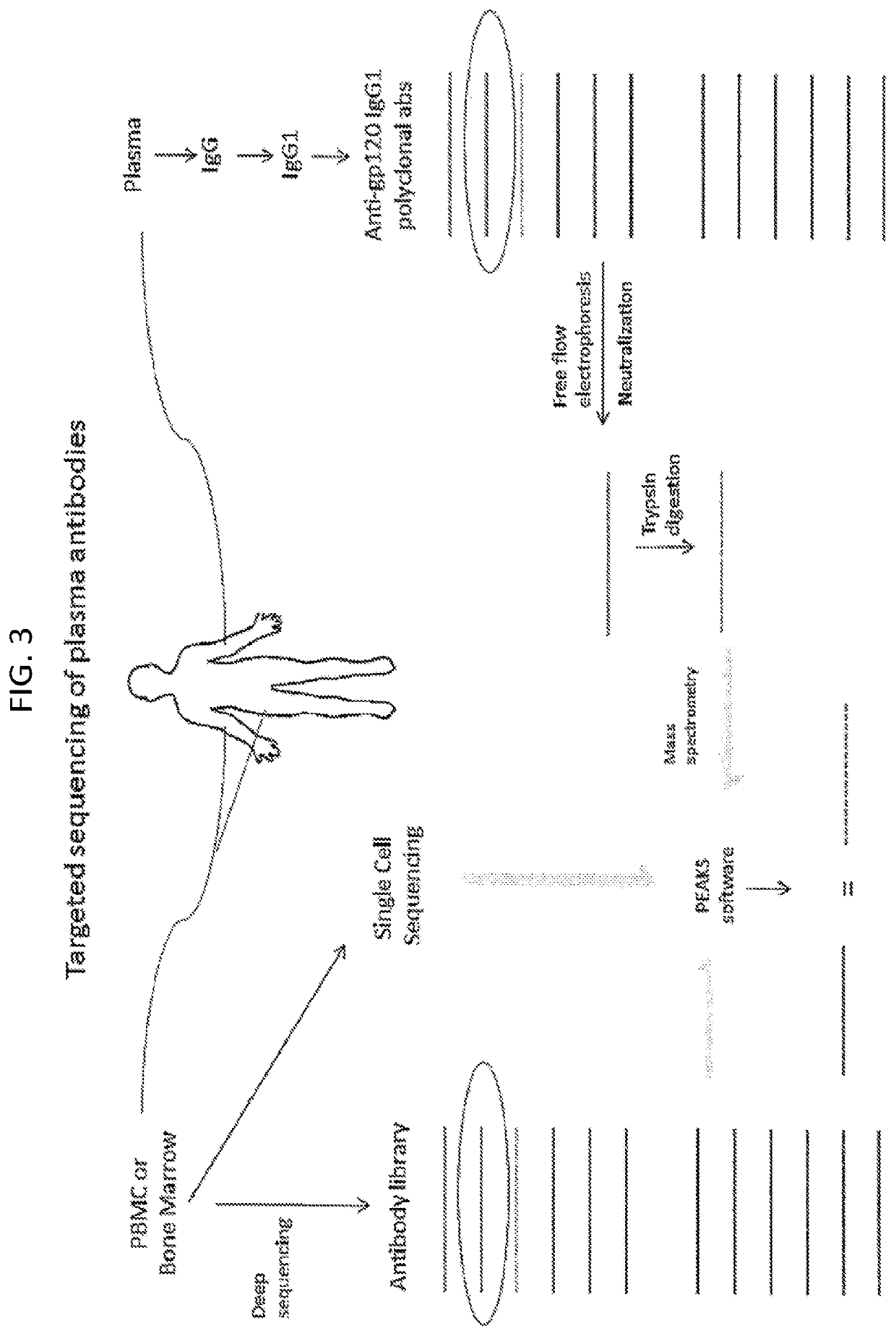
Methods of making active antibodies from biological fluids
ActiveUS20200182883A1Immunoglobulins against virusesPeptide preparation methodsAntibody fragmentsBlood plasma
The present invention provides a method of making an antibody by identifying a circulating antibody with activity from a subject comprising i) subjecting biological fluid selected from the group consisting of blood, plasma and serum and combinations thereof from the subject to one or more rounds of affinity chromatography to purify the circulating antibody; ii) optionally further subjecting the circulating antibody to isoelectric focusing to purify the circulating antibody based on charge; iii) testing the purified circulating antibody for activity; iv) digesting the purified circulating antibody from parts i) or ii) to create an antibody fragment; v) subjecting the antibody fragment to mass spectrometry to generate a mass assignment and a deduced amino acid sequence of the antibody fragment; vi) comparing the deduced amino acid sequence with an amino acid sequence of an antibody generated from the subject's B-cells to identify an antibody sequence that matches the deduced amino acid sequence; vii) generating an antibody comprising light chain and heavy chain CDR sequences of the B-cell antibody that matches the deducted amino acid sequence of party vi); and viii) testing the antibody of part vii) for activity.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Processes and products with modified antibodies of human milk fat globule specificity
InactiveUS20060216303A1Reduce concentrationIn-vivo radioactive preparationsPeptide/protein ingredientsVaccinationHybrid vector
An analogue peptide that comprises the variable regions of the light or heavy chains of an antibody of a first species selectively binding to a carcinoma antigen has 1 to 46 amino acids of the framework regions per chain substituted with amino acids such as those present in equivalent positions in antibodies of a species other than the first species, or fragments thereof comprising 1 to 3 variable region CDRs per chain and optionally flanking regions thereof of 1 to 10 or more amino acids, alone or with an N-terminal fragment of 1 to 10 or more amino acids, combinations or mixtures thereof. The polypeptide may also comprise an effector agent and / or be glycosylated, and is presented as a composition with a carrier. The analogue peptides are used in diagnostic kits for carcinomas and methods for in vivo imaging and treating a primary or metastasized carcinoma, and in vitro diagnosing a carcinoma, ex vivo purging neoplastic cells from a biological fluid. RNAs and DNAs encode the analogue peptide, and a hybrid vector carrying the nucleotides and transfected cells express the peptides and a method produces the analogue peptide. An anti-idiotype polypeptide comprises polyclonal antibodies raised against an anti-carcinoma antibody or the analogue peptide of this invention, monoclonal antibodies thereof, Fab, Fab′, (Fab′)2, CDR, variable region, or analogues or fragments thereof, combinations thereof with an oligopeptide comprising a TRP trimer, tandem repeats thereof, or combination or mixtures thereof. An anti-idiotype hybrid polypeptide with an effector agent and the anti-idiotype polypeptide, an anti-carcinoma vaccine, an anti-carcinoma vaccination kit, a method of vaccinating against carcinoma and a method of lowering the serum concentration of a circulating antibody or polypeptide are provided.
Owner:DO COUTO FERNANDO +3
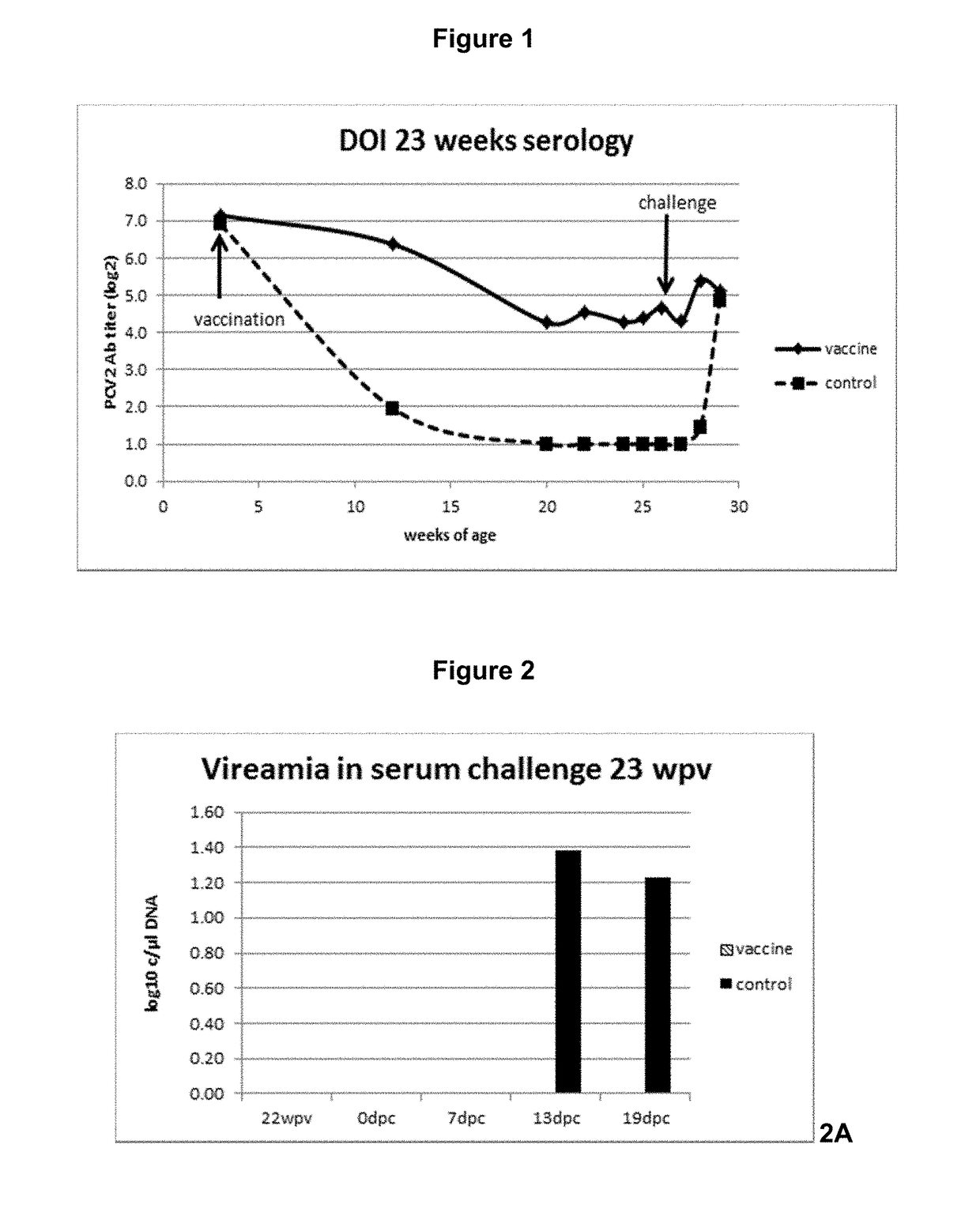
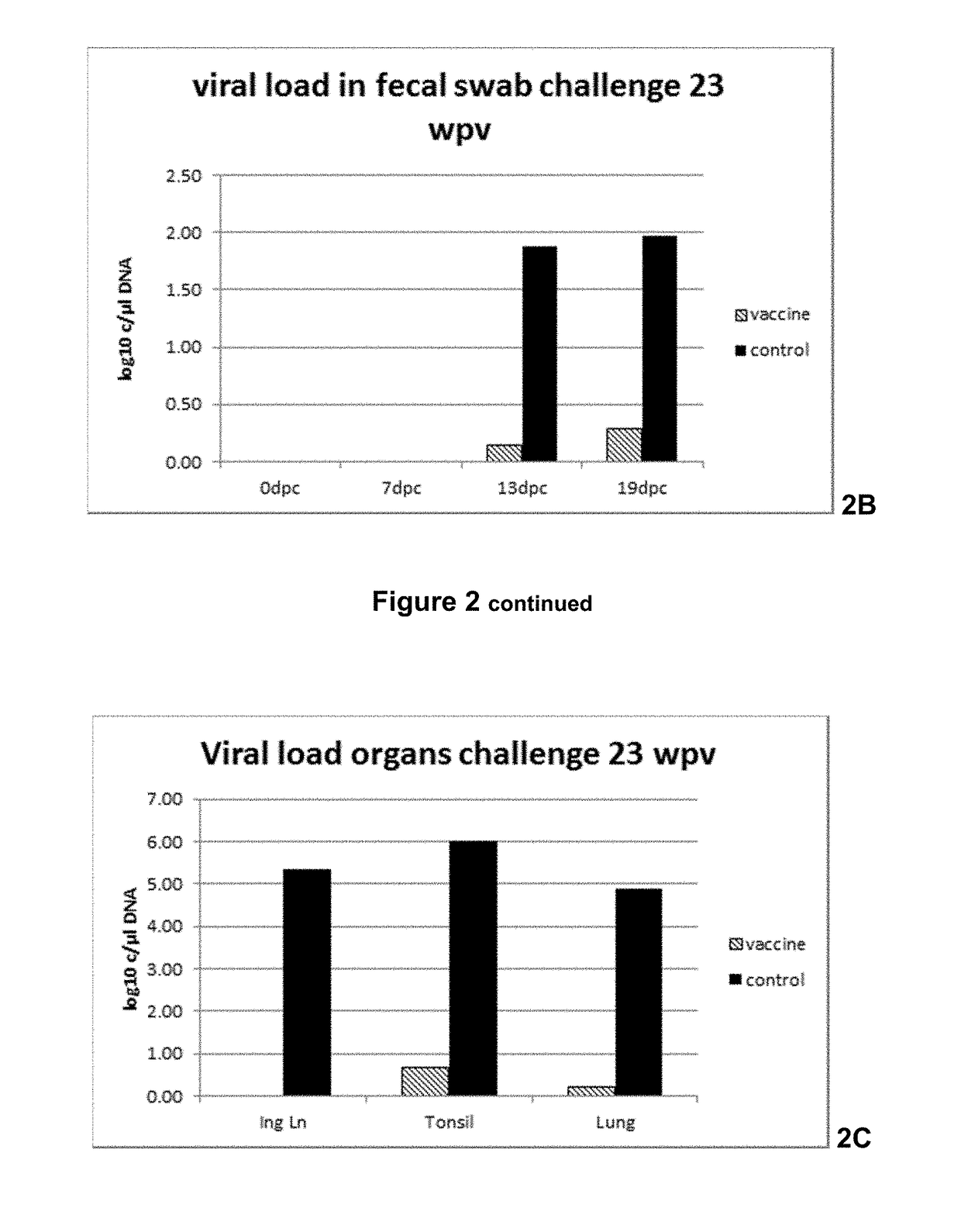
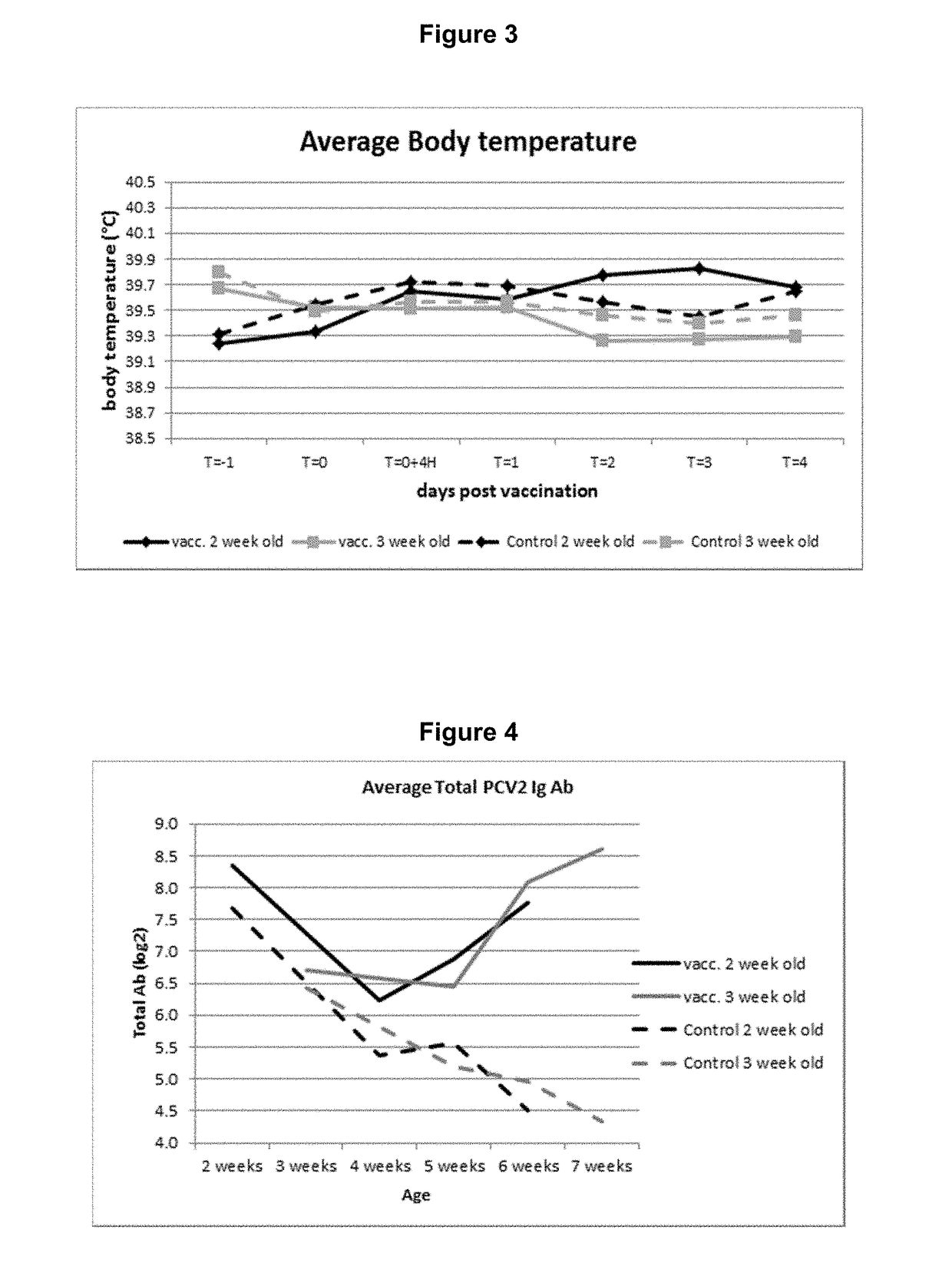
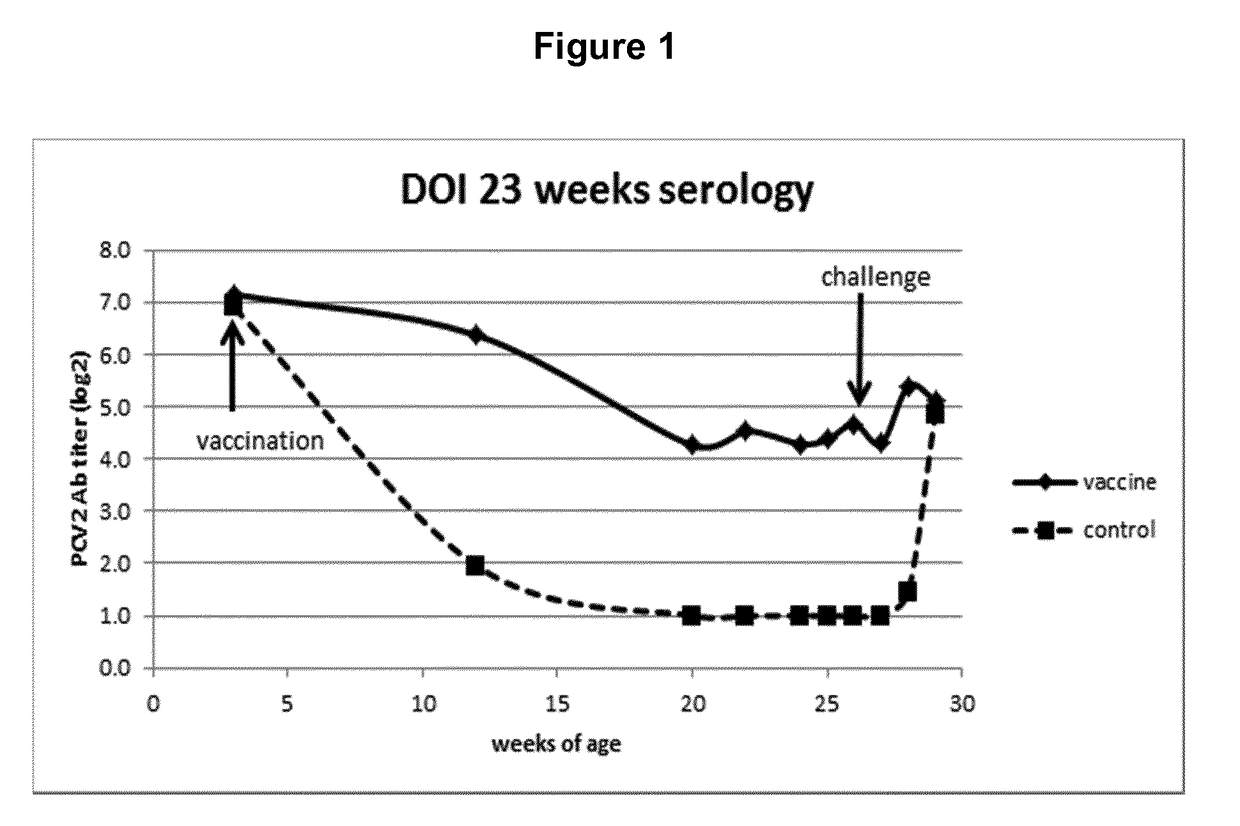
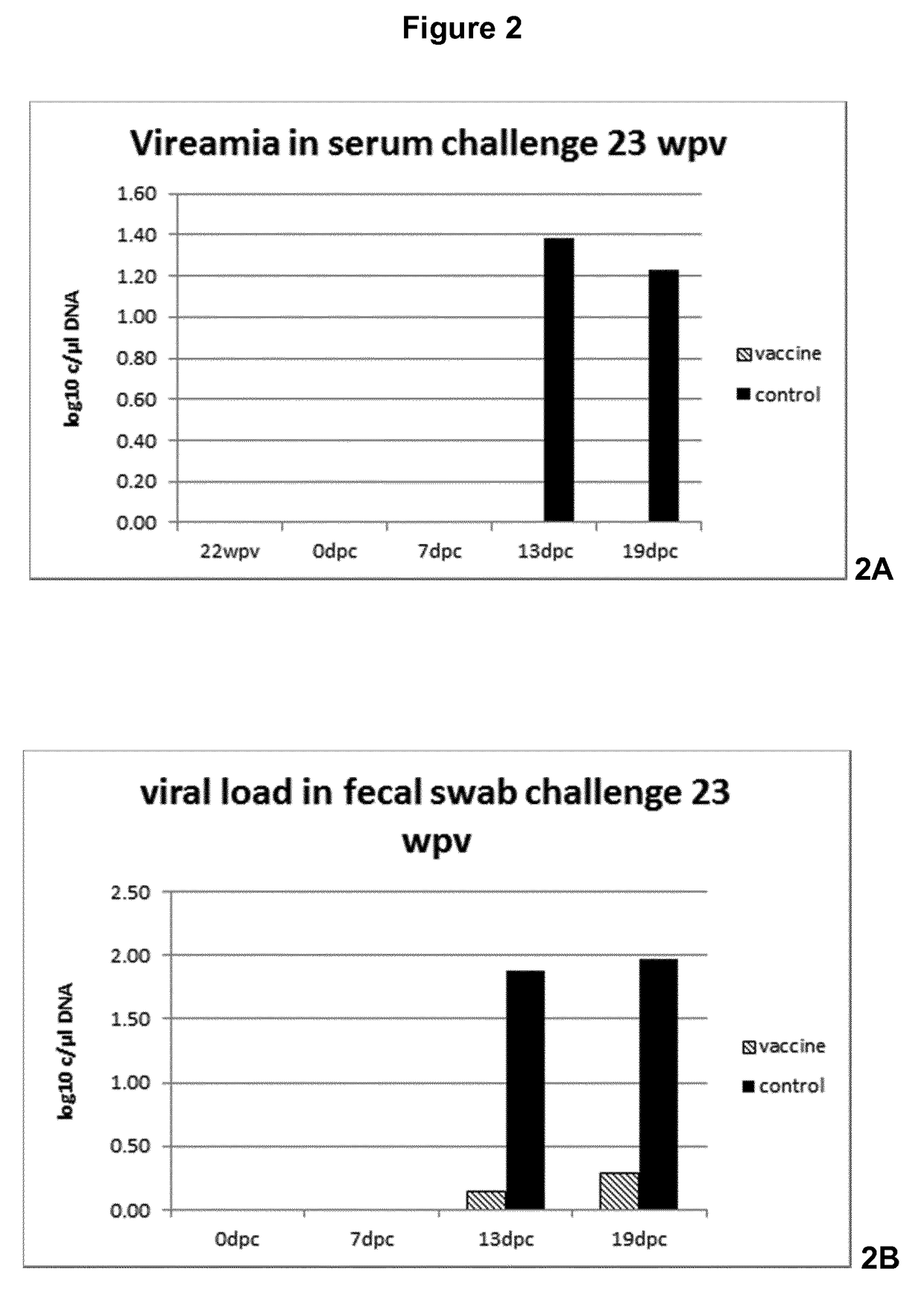
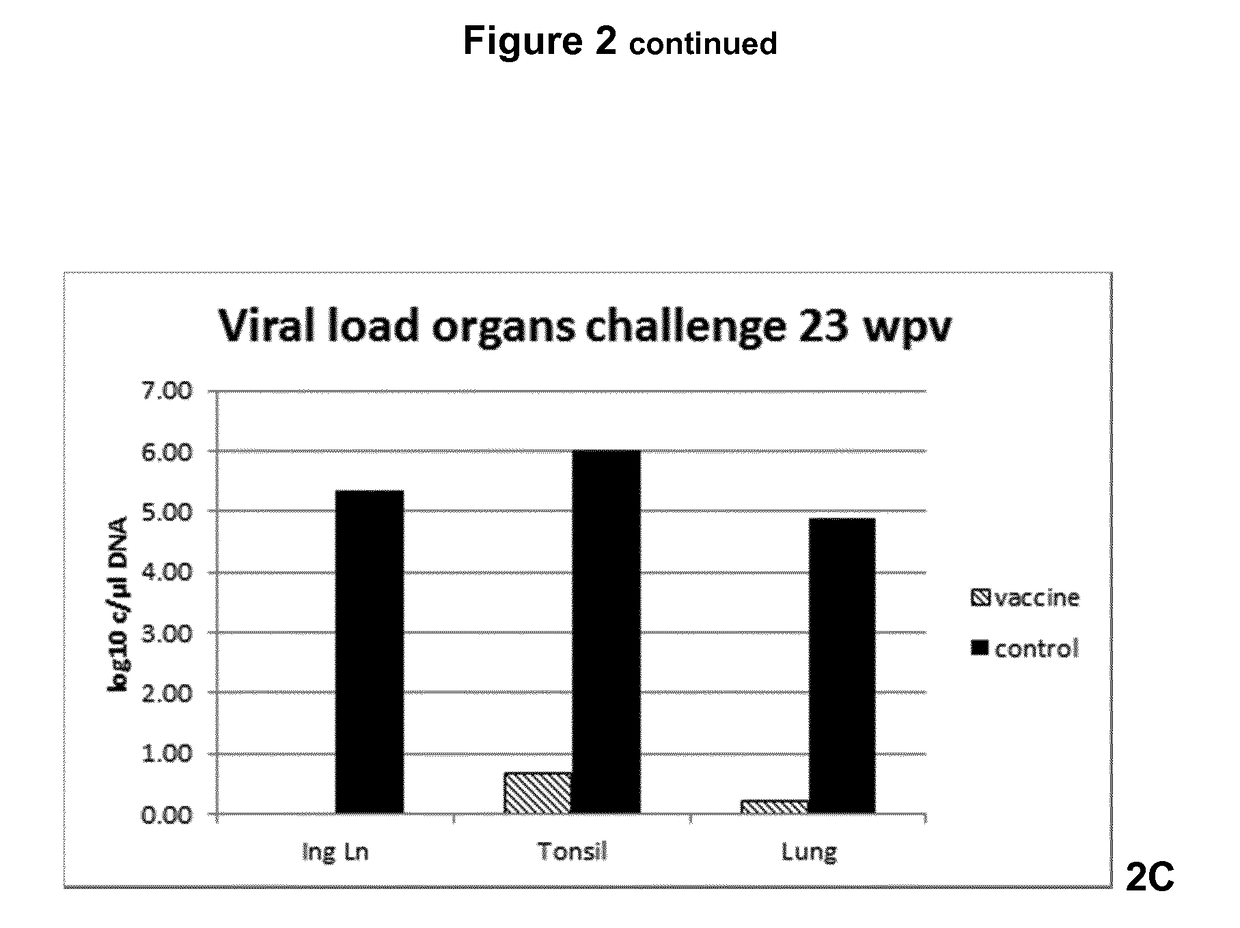
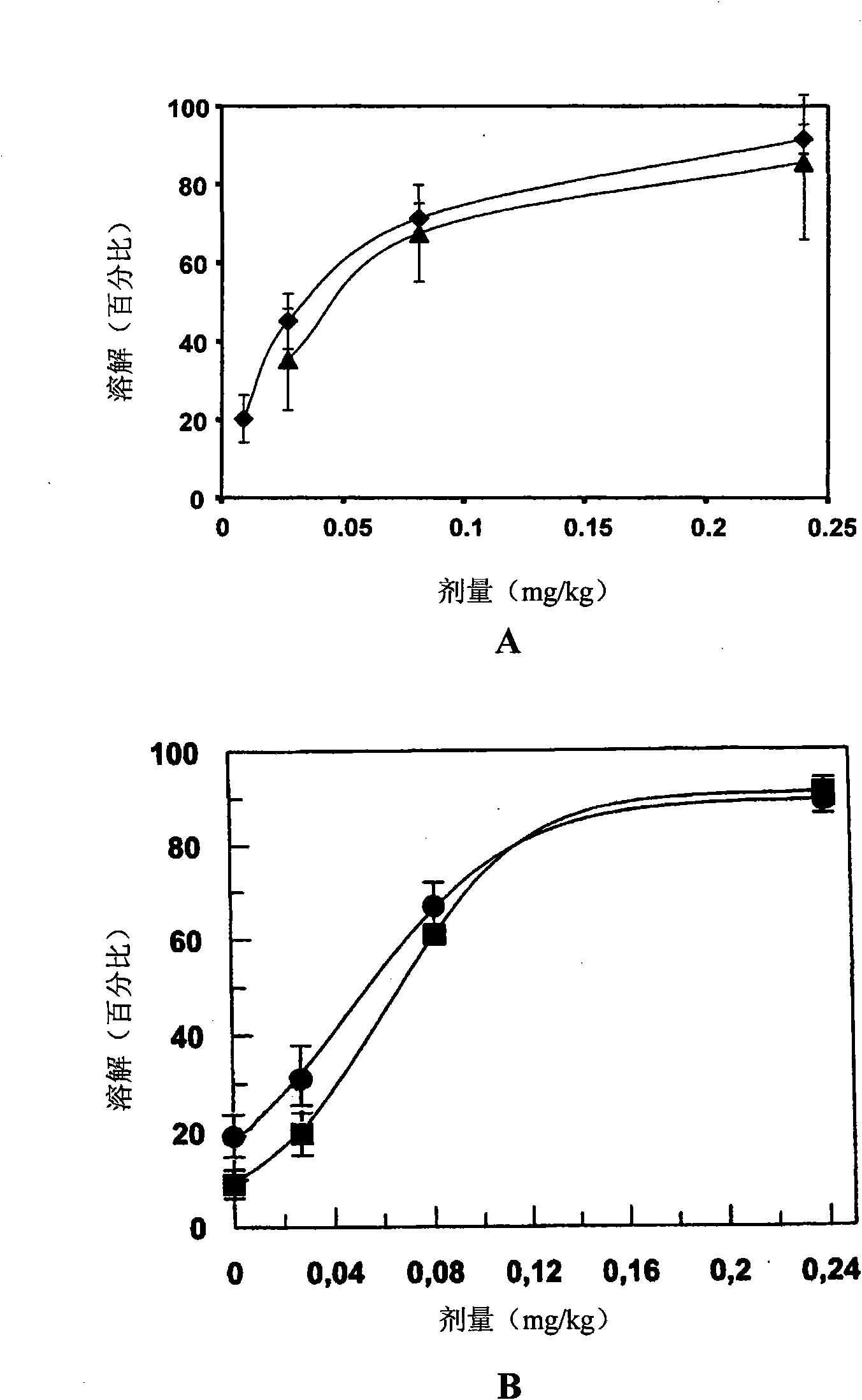
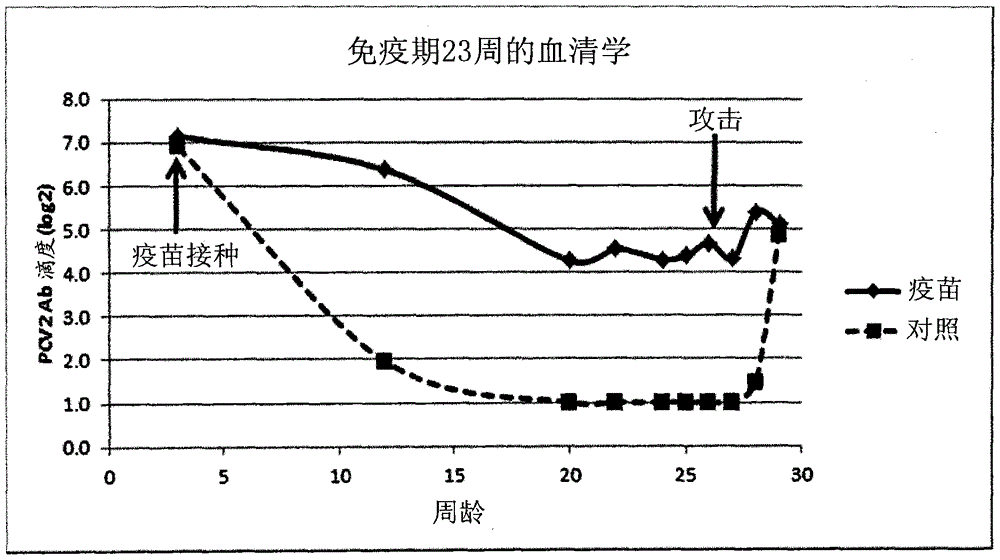
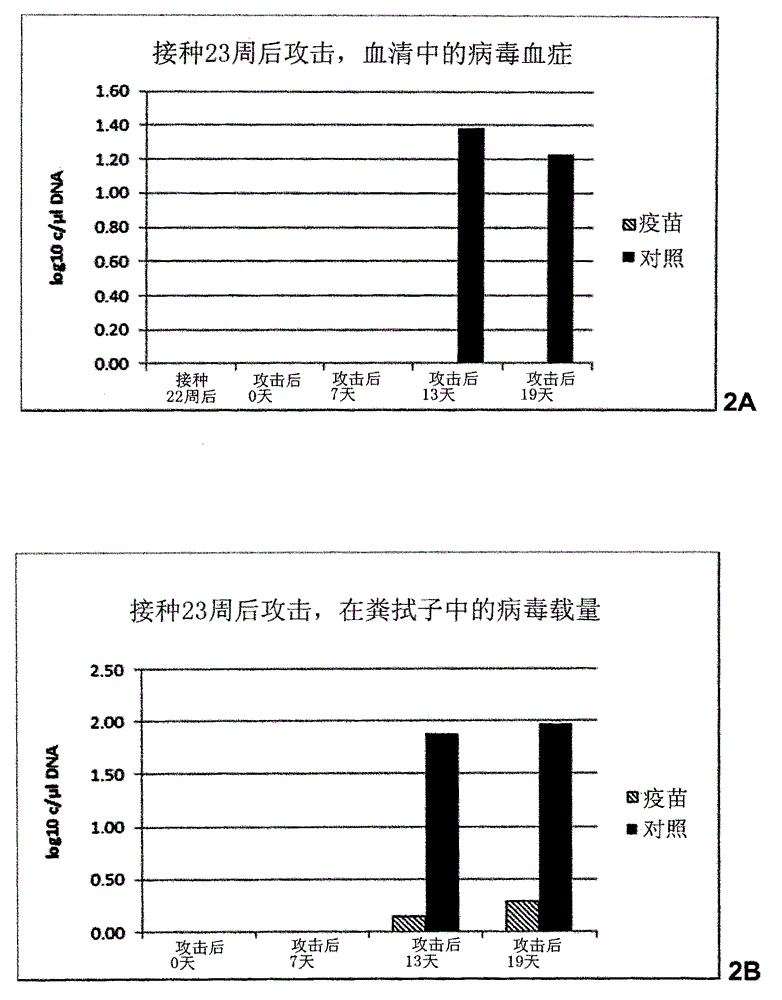
Vaccine against porcine circo virus type 2
Owner:INTERVET INC
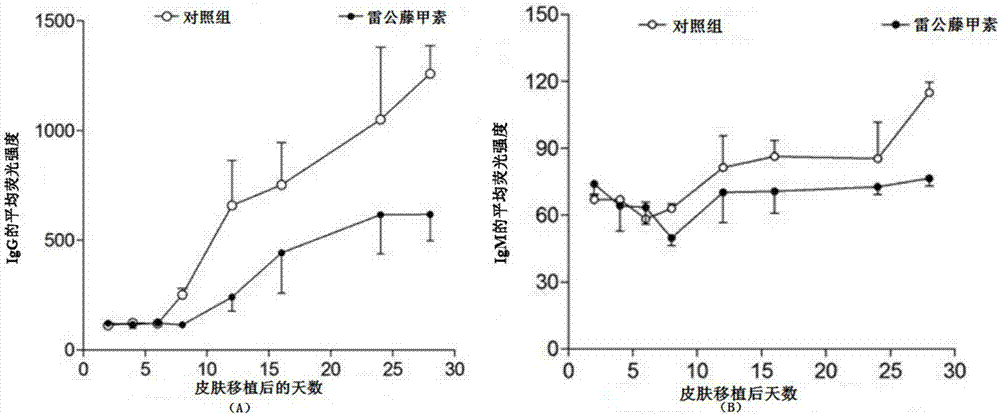
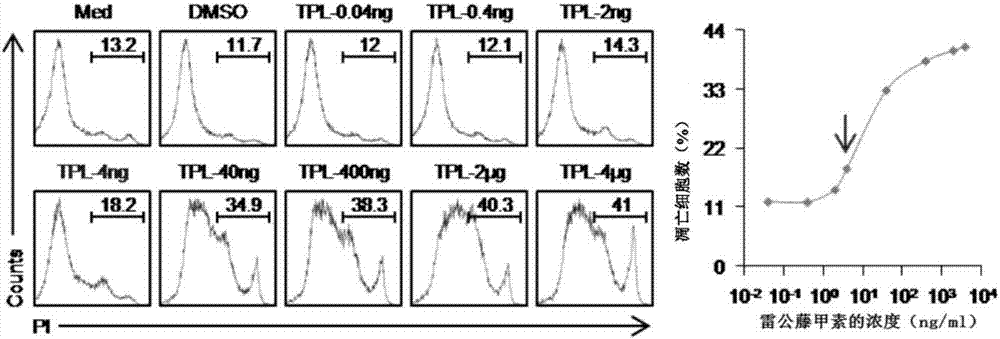
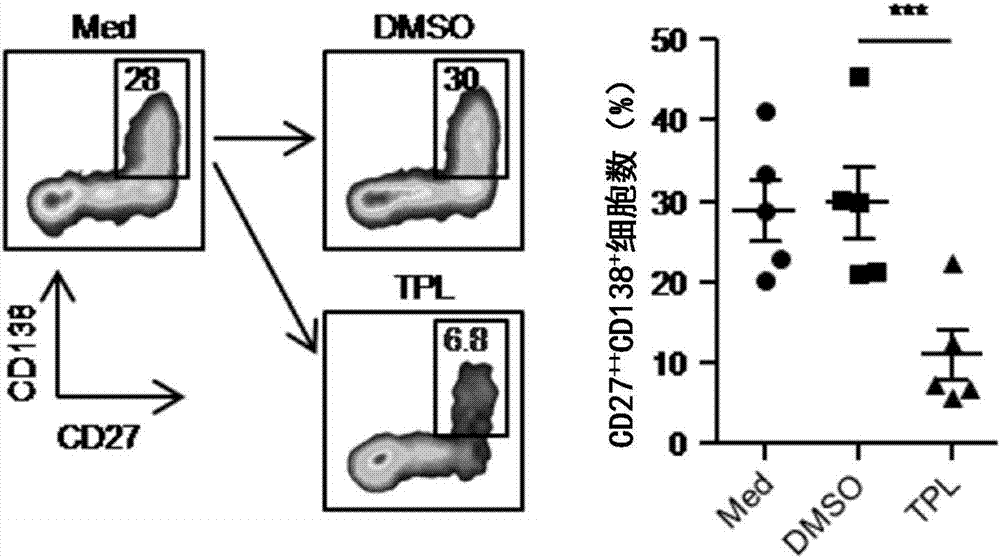
Triptolide and application of modifier thereof to inhibition of antibody generation by B cells
InactiveCN106890187AInhibitionOrganic active ingredientsImmunological disordersSkin graftingTriptolide
The invention discloses triptolide and application of a modifier thereof to inhibition of antibody generation by B cells. The inventor utilizes triptolide to treat mice sensitized caused by skin grafting of different strains, it is found that triptolide can inhibit generation of a circulating antibody DSA, and an in vitro experiment is further adopted to verify that triptolide can inhibit antibody generation by the B cells. The invention discloses a new application of triptolide. It is verified that triptolide and the modifier thereof can inhibit antibody generation by the B cells, and the basis can be provided for preparing medicines for inhibit antibody generation by the B cells.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Methods of making active antibodies from biological fluids
The present invention provides a method of making an antibody by identifying a circulating antibody with activity from a subject comprising i) subjecting biological fluid selected from the group consisting of blood, plasma and serum and combinations thereof from the subject to one or more rounds of affinity chromatography to purify the circulating antibody; ii) optionally further subjecting the circulating antibody to isoelectric focusing to purify the circulating antibody based on charge; iii) testing the purified circulating antibody for activity; iv) digesting the purified circulating antibody from parts i) or ii) to create an antibody fragment; v) subjecting the antibody fragment to mass spectrometry to generate a mass assignment and a deduced amino acid sequence of the antibody fragment; vi) comparing the deduced amino acid sequence with an amino acid sequence of an antibody generated from the subject's B-cells to identify an antibody sequence that matches the deduced amino acid sequence; vii) generating an antibody comprising light chain and heavy chain CDR sequences of the B-cell antibody that matches the deducted amino acid sequence of party vi); and viii) testing the antibody of part vii) for activity.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Vaccine against porcine circo virus type 2
ActiveUS20170157236A1Different antibody responseReduce loadAntibacterial agentsBacterial antigen ingredientsVirus typeProphylactic treatment
The present invention pertains to a vaccine comprising a non-replicating immunogen of porcine circo virus type 2 for use in prophylactically treating an animal that has circulating antibodies directed against porcine circo virus type 2, against an infection with pathogenic porcine circo virus type 2 by a single dose administration of the vaccine into the dermis of the animal.
Owner:INTERVET INC
Production method of eggs with high immune globulin content
The invention provides a novel technique and a novel method for producing novel health care food, i.e. a technique and a method for producing health care eggs with high immune globulin content by biological nanometer materials. The invention is characterized in that compound feed which supplies nutrition is designed and prepared according to the nutrition physiology characteristics of the immune system of a laying hen to be used as base effect substance, organic atomic state nano selenoprotein is used as a main effect nutrition factor by the special biology effect, iodine from feed-grade potassium iodide and escherichia coli vaccine immune are combined to be used as auxiliary effect factors for cooperating with the effect of nano-selenium so as to fully stimulate the function of the immune system of the laying hen, improve the circulating antibody level of the hen, increase the antibody deposition in an egg secretion process and form eggs with high immune globulin content. With the method and the technique, the immune globulin content of the egg can be increased by more than 36%. The contribution rate of the synergy of the invention to the immune globulin output of the egg can reach more than 62%.
Owner:福建省新闽科生物科技开发有限公司
Method of modifying the immune response
InactiveUS20110229511A1Reduce riskOrganic active ingredientsCarrier-bound antigen/hapten ingredientsResponse methodIncompatible blood transfusion
Owner:HENRY STEPHEN MICHAEL +1
In vitro assay for quantitating secreted antibodies in lymphocyte supernatant for evaluation of vaccine or antigen induced specific antibody secretion from ex vivo circulating antibody-secreting lymphocytes
InactiveUS20150268225A1Immunoglobulins against bacteriaBiological testingAntibody secretionLymphocyte
The present invention provides methods of quantitating recent secreted antigen specific antibodies from supernatant of antibody secreting cells (ASC) in vitro culture for evaluation of vaccine or antigen induced antigen specific antibody secretion without ex vivo antigen stimulation.
Owner:CHANG HUI SUNNY
Immunological modulation of insulin-like growth factor 1 for cancer prevention/treatment and prolonging longevity
ActiveUS20080187536A1Reduce the incidence of cancerExtend your lifeAntibacterial agentsOrganic active ingredientsPassive ImmunizationsInsulin-like growth factor
Provided is a simple and safe immunization procedure to reduce cancer incidence and increase longevity by modulating IGF-1 levels in the body. Described are cancer preventive vaccines and methods that elicit circulating antibodies specific to insulin-like growth factor 1 (IGF-1) in the body. Many cancers will be less likely to occur and spread in the absence or reduced levels of the stimulatory signals provided by IGF-1. Longevity also can be extended by immunologically lowering the level of bioavailable IGF-1 in adult animals. This prolongation of lifespan resulting from lower IGF-1 levels is produced independently of the inhibitory effects on carcinogenesis. However, the two IGF-1-mediated processes are linked mechanistically. Methods of active and passive immunization to suppress IGF-1 activity are included. Also described are methods for increasing longevity or reducing one or more symptoms of aging in a warm-blooded animal comprising administering anti-IGF-1 antibodies such that IGF-1 is inactivated or suppressed or administering IGF-1 antigen such that the animal produces endogenous antibodies to IGF-1.
Owner:RASO VICTOR DR
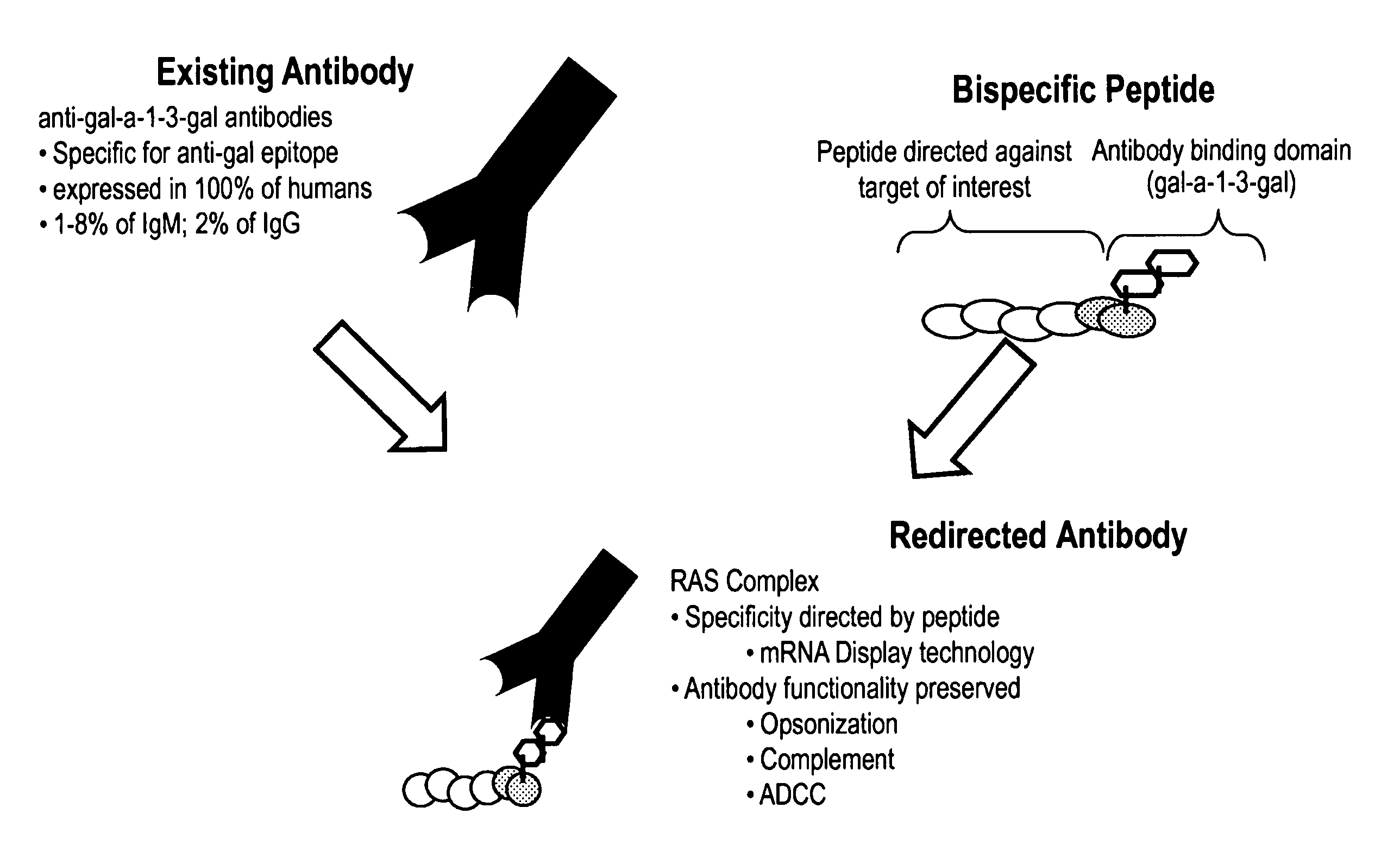
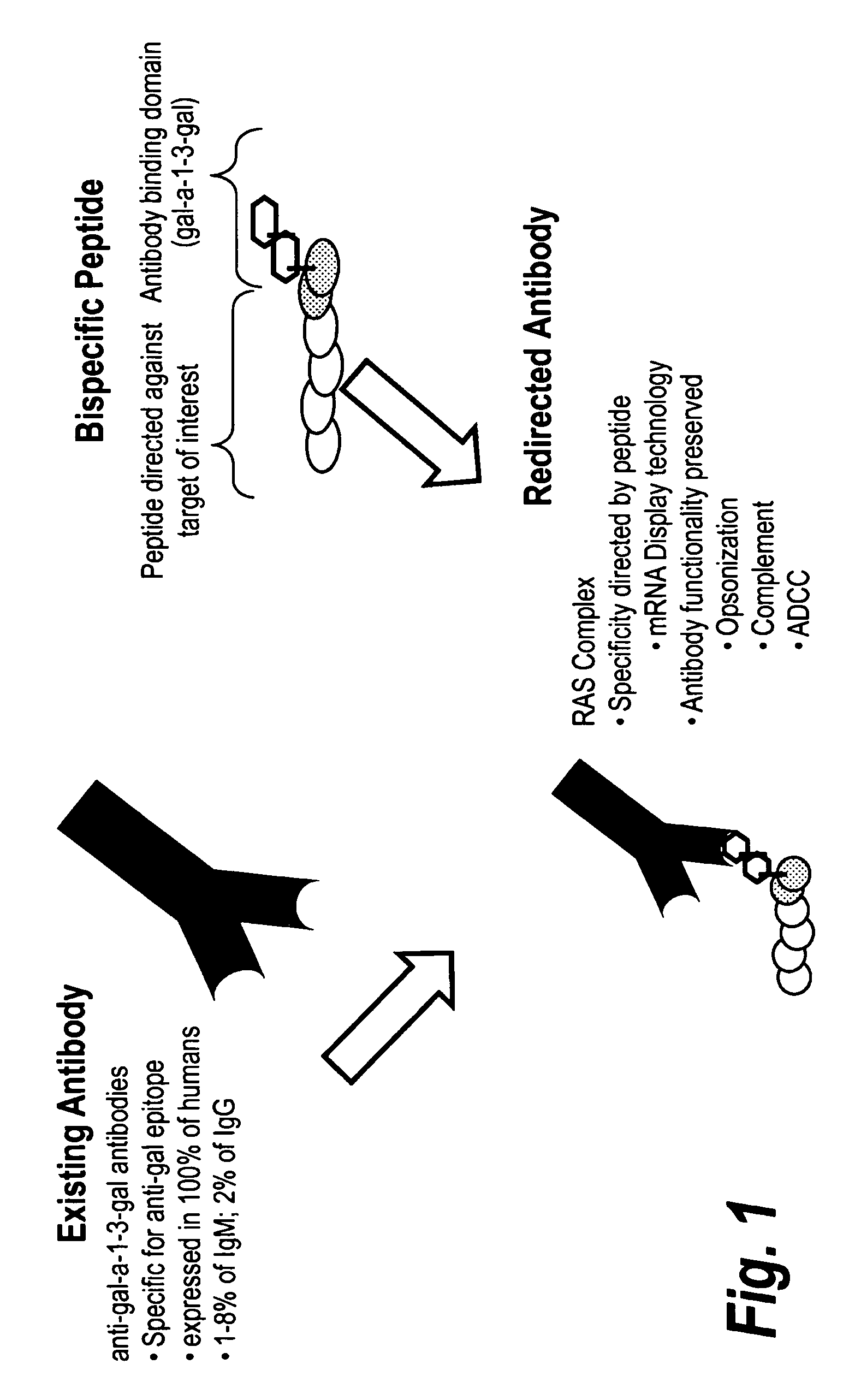
High affinity adaptor molecules for redirecting antibody specifity
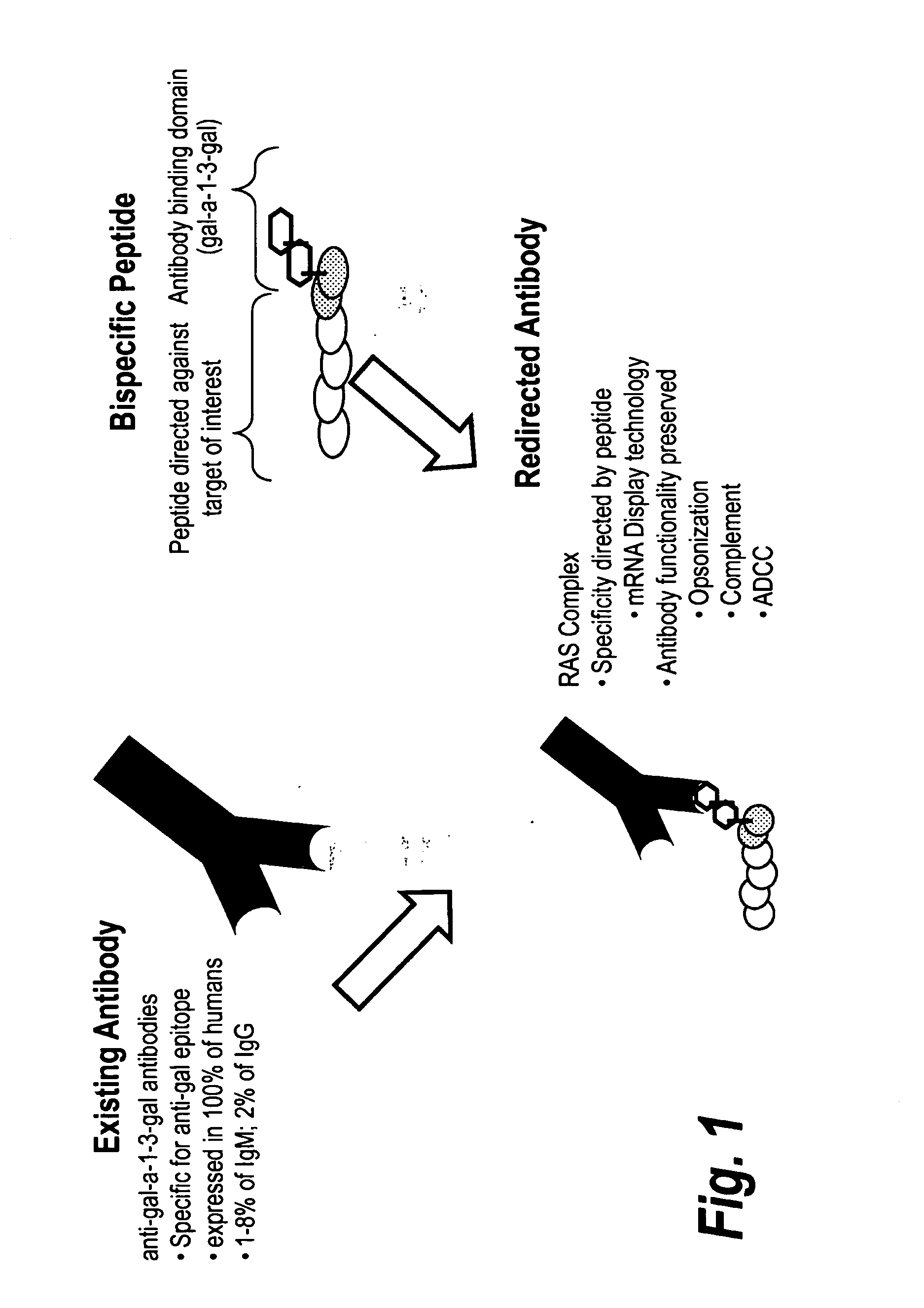
InactiveUS9284548B2High binding affinity and selectivityEffective redirectingLibrary screeningTripeptide ingredientsAdaptor moleculeBiochemistry
Owner:OPSONIC THERAPEUTICS
Autoantibodies for protein antigens as markers for cancer of gingivo-buccal complex
ActiveUS8492100B2Inhibit tumor cell growthFacilitate killingMammal material medical ingredientsBiological testingCellular antigensCancer cell
The present invention relates to identification of a set of proteins, which elicit an autoantibody response in patients with cancer of gingivo-buccal complex. Systematic comparisons of serum samples from clinically normal individuals and from patients with cancer of gingivo-buccal complex has revealed significant differences in the presence of autoantibodies in sera against cellular antigens present in a cancer cell line. The autoantibody response to a single or combination of these protein antigens serves as a novel marker and can be utilized for screening, early detection, prognosis, and potential target for therapy. The invention also provides for the use of the identified protein antigens in immunoassays designed to detect the presence of serum antibodies to the specific protein antigens in sera from individuals that harbor such antibodies. The invention also relates to the use of the identified antigens as immunogens for stimulation of an immune response in patients expressing such protein antigens. The invention is demonstrated by way of example in which elevated levels of circulating antibodies reactive against tumor specific antigens were identified in sera derived from patients with cancer of gingivo-buccal complex. The utility of identified antigens for early detection is assessed by analysis of sera from patients with leukoplakia of gingivo-buccal complex.
Owner:COUNCIL OF SCI & IND RES
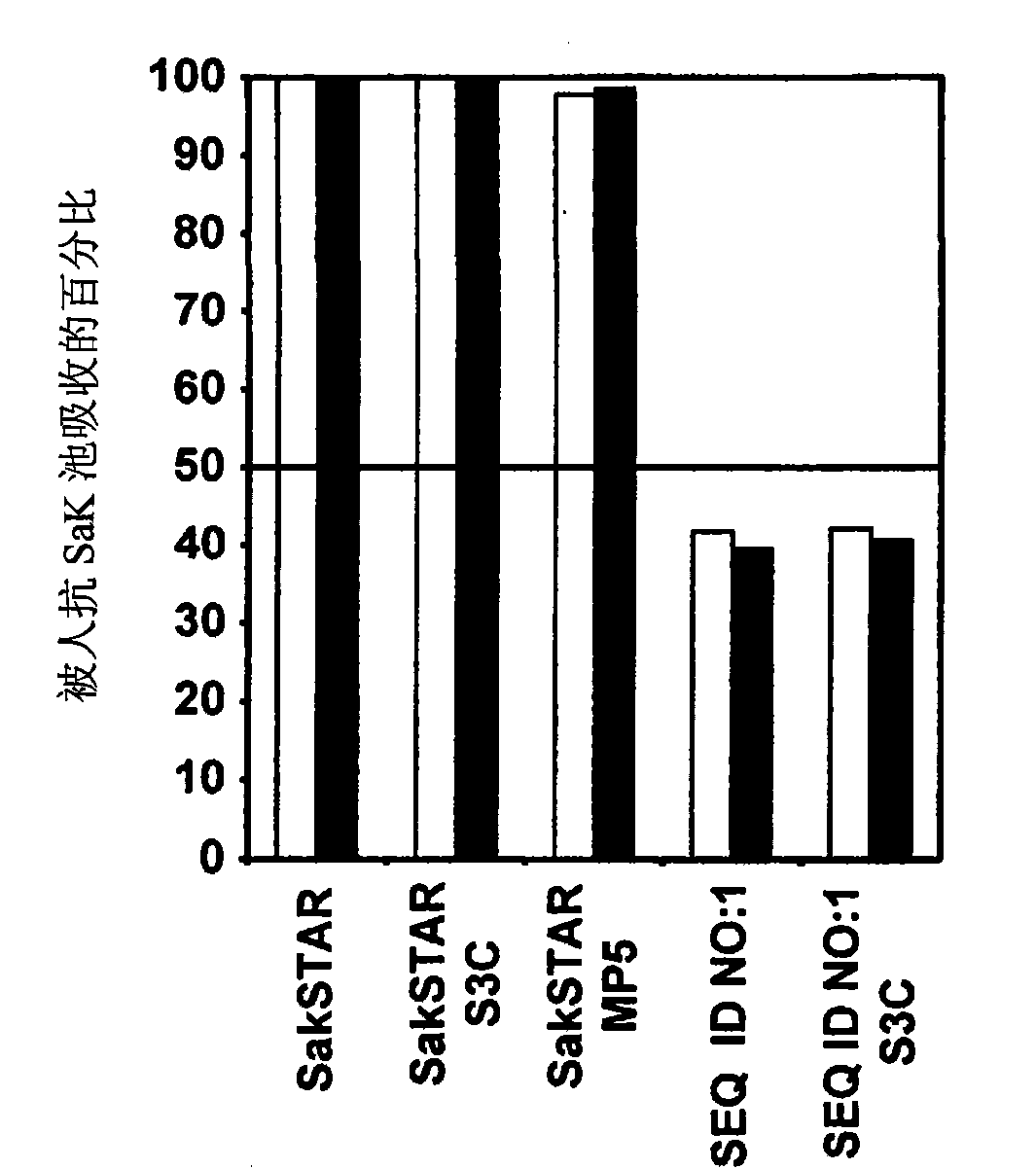
Staphylokinase variant
The present invention relates to an optimized thrombolytically or fibrinolytically active staphylokinase variant exhibiting low T-cell immunogenicity and reduced clearance by circulating antibodies that can be expressed at high levels.
Owner:凝血基因公司
Method of modifying the immune response
ActiveUS20160030541A1Reduce riskOrganic active ingredientsCarrier-bound antigen/hapten ingredientsResponse methodCvd risk
Owner:KODE BIOTECH
Vaccine against porcine circo virus type 2
InactiveCN105848675AAntibacterial agentsBacterial antigen ingredientsVirus typeProphylactic treatment
The present invention pertains to a vaccine comprising a non-replicating immunogen of porcine circo virus type 2 for use in prophylactically treating an animal that has circulating antibodies directed against porcine circo virus type 2, against an infection with pathogenic porcine circo virus type 2 by a single dose administration of the vaccine into the dermis of the animal.
Owner:INTERVET INT BV
Method of modifying the immune response
ActiveUS10124047B2Reduce riskOrganic active ingredientsSnake antigen ingredientsResponse methodIncompatible blood transfusion
Owner:KODE BIOTECH
High affinity adaptor molecules for redirecting antibody specifity
InactiveUS20120271033A1High binding affinityHigh selectivityLibrary screeningPeptide sourcesAdaptor moleculeBiochemistry
Owner:OPSONIC THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com