Staphylokinase variant
A technology of staphylokinase and variants, applied in the fields of peptide/protein components, peptides, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In order to prepare modified staphylokinase variants, the above preparation method may further include the following steps:
[0040] (iii) modifying the staphylokinase variant isolated in (ii).
[0041] In one embodiment, the step of modifying the staphylokinase variant comprises pegylation of the staphylokinase molecule.
[0042] All suitable hosts or host cells and accompanying host cell culture media are contemplated. Non-limiting examples of host cells include: Escherichia coli, Saccharomyces (such as Saccharomyces cerevisiae), Schizosaccharomyces (such as S. pombe), Hansenula (such as polymorpha), insect cells (such as Spodoptera frugiperda) And mammalian cells (such as COS cells, CHO cells).
Embodiment
[0044] Staphylokinase variant preparation
[0045] Molecular cloning, expression, (recombinant) production and purification of staphylokinase and variants thereof were performed essentially as described in detail in any one of EP0525252A1, WO 93 / 13209, WO 96 / 21016 and WO 99 / 40198.
[0046] In vitro fibrinolytic / thrombolytic activity
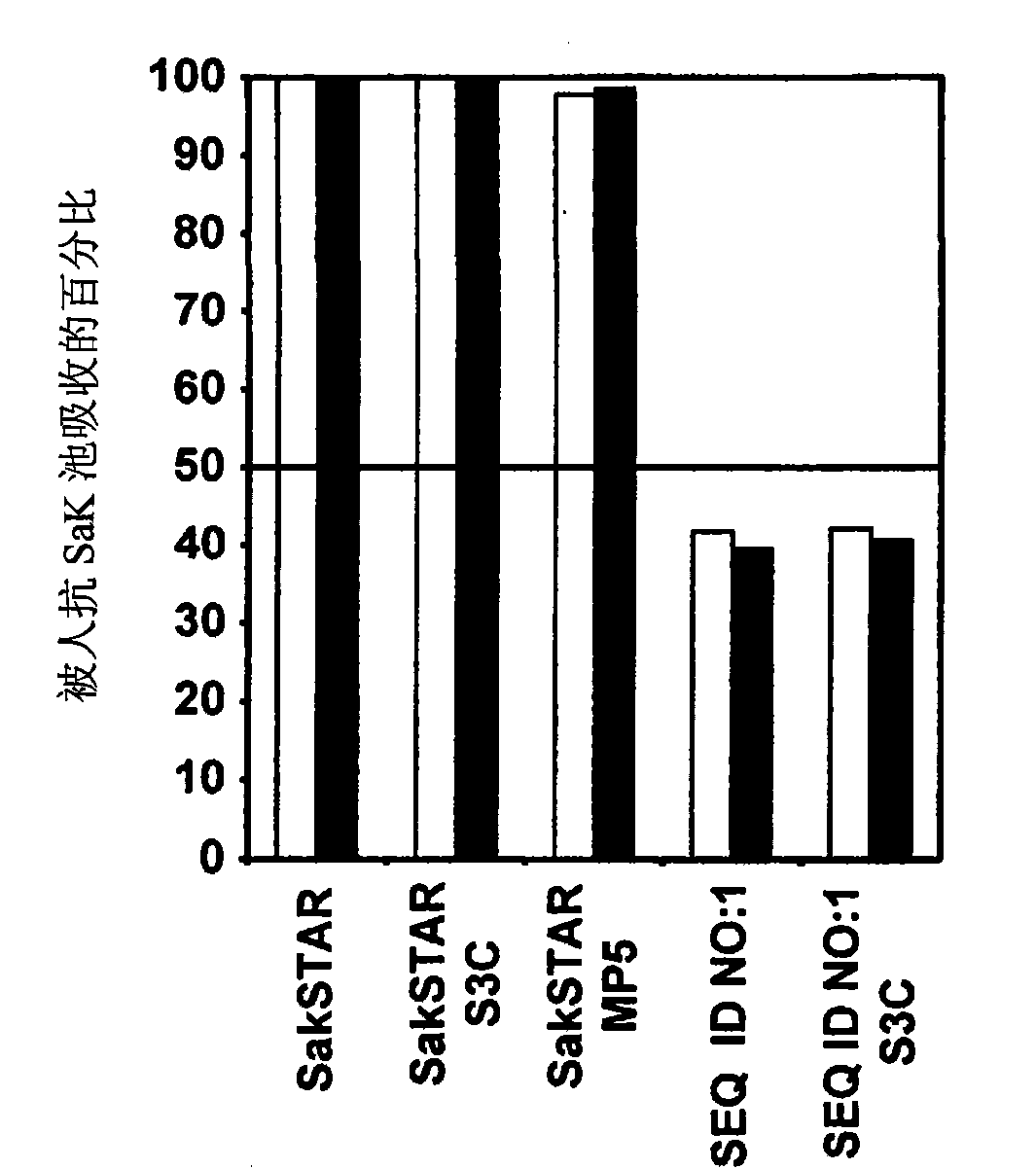
[0047] The specific activity of the staphylokinase shown in SEQ ID NO: 1 determined in the chromogenic thrombolytic assay was 55±15 kU / mg, which was much lower than that of wild-type SakSTAR (153±33 kU / mg). Chromogenic analysis was performed essentially as described in Example 2.5 of WO 99 / 40198 using S2403 as the chromogenic substrate.
[0048] In contrast, the concentration required for staphylokinase shown in SEQ ID NO: 1 to induce 50% clot lysis in human plasma is similar to the concentration of SakSTAR required (the concentration of SEQ ID NO: 1 is 360 ± 30 ng / mL and the concentration of SakSTAR is 343±35ng / mL). The assay was performed es...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com