Application of fluorine-containing compound modified cationic polymer in preparation of vaccine drugs
A technology of cationic polymers and compounds, which is applied in the fields of polymer chemistry and medical biomaterials, and can solve problems such as the reduction of vaccination efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: prepare the chitosan (deacetylation degree ≥ 95%, viscosity 100-200mpa.s) of the different degree of modification of perfluoroheptanoic acid, wherein the molar ratio of perfluoroheptanoic acid and N-glucosamine unit is respectively 1: 2.2, 1:4.2, 1:8.4, 1:16.8.
[0072] Synthesis method: (1) Preparation of chitosan acetic acid aqueous solution: take 200 mg of fully dried chitosan and add it to 10 ml of 1% acetic acid aqueous solution, stir for 30 min to fully dissolve, then slowly add 1.6 ml of 0.5 M sodium hydroxide dropwise, stir Until the solution is clear and the pH is around 6.5. Prepare 4 parts of chitosan acetic acid aqueous solution in this way. (2) Activation of perfluoroheptanoic acid (13 fluoroheptanoic acid): Weigh 206mg, 103mg, 51.5mg, and 26mg of perfluoroheptanoic acid respectively, dissolve them in an appropriate amount of anhydrous dimethyl sulfoxide, and add appropriate amount of EDC in turn , NHS in the dark and stirred for 1h. (3) Pr...
Embodiment 2
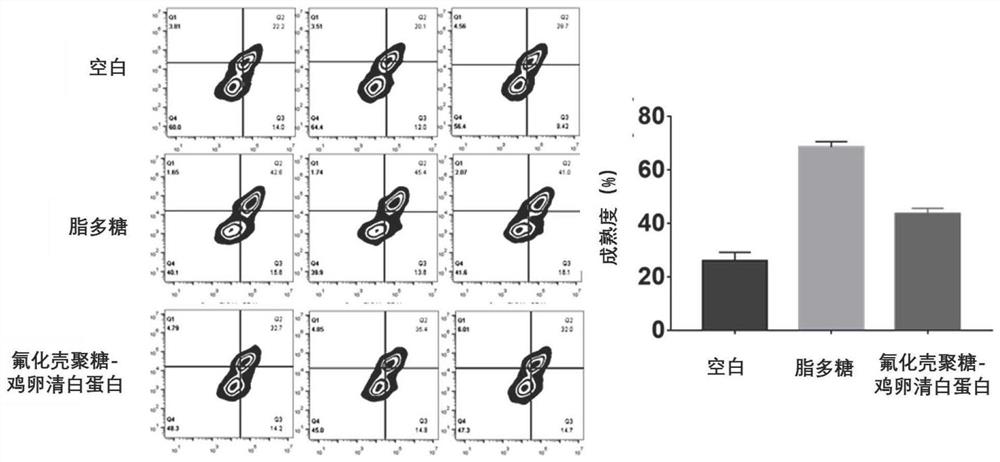
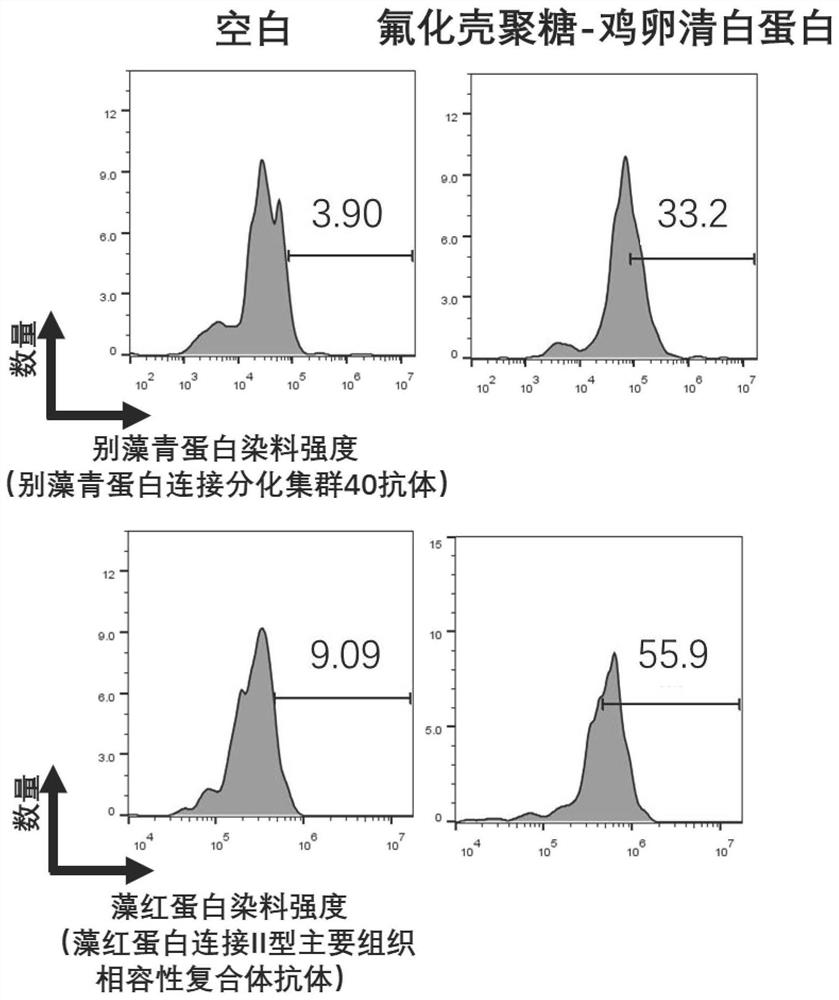
[0074] Example 2: 1. Fluorinated chitosan-chicken ovalbumin complexes were prepared with perfluoroheptanoic acid-modified chitosan as a carrier, incubated with bone marrow-derived dendritic cells, and investigated to stimulate the maturation of dendritic cells by the complexes Ability.
[0075] specific method:
[0076] Preparation of perfluoroheptanoic acid-modified chitosan-chicken ovalbumin complex: Weigh 0.9 mg of perfluoroheptanoic acid-modified chitosan and dissolve it with 900 μL of ultrapure water, and drop 100 μL (20 mg / mL) of chicken ovalbumin, and continued to stir for one hour to obtain a perfluoroheptanoic acid-modified chitosan-chicken ovalbumin (FCS-OVA) complex.
[0077] Add 10 μL of the above-prepared complex to a 24-well plate, and add 1 mL of cell suspension containing 1 million dendrites. Incubate in a 37°C incubator for 24 hours, stain the dendritic cells with FITC-CD11c, PE-CD86, and APC-CD80, analyze the fluorescence signal of FITC with flow cytometry...
Embodiment 3
[0096] Embodiment 3: prepare the chitosan (deacetylation degree ≥ 95%, viscosity 100-200mpa.s) of the different modification degree of 3-fluorobenzoic acid, wherein the feeding molar ratio of 3-fluorobenzoic acid and N-glucosamine unit is respectively 1:2.1, 1:4.2, 1:8.4, 1:16.8.
[0097] Synthetic method: (1) Preparation of chitosan acetic acid aqueous solution: Weigh 200 mg of fully dried chitosan and add it to 10 ml of 1% acetic acid aqueous solution. Of course, hydrochloric acid aqueous solution can also be used. Stir for 30 minutes to fully dissolve, then slowly add 1.6 ml of 0.5 M sodium hydroxide, stirred until the solution was clear and the pH was around 6.5. Simply consider the angle sodium hydroxide of alkalization solution and can be replaced by alkalis such as ammoniacal liquor, triethylamine, but the by-product that uses sodium hydroxide is sodium chloride from the product process angle, is more suitable for industrialization. Prepare 4 parts of chitosan acetic a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com