Block copolymers based on naphthalene diimide and indacene cyanindanone based on the main chain structure and their applications in organic photovoltaic devices
A technology for introducing and saving cyanoindanone and naphthalene diimide, which is applied in the field of polymer photoelectric materials, can solve the problems of uncontrollable phase separation and low absorption coefficient of active layer films, and achieves high performance and high absorption coefficient. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthetic route see Figure 7 ~ Figure 8 .
[0041] (1) Monomers M1, M2, and M3 were synthesized according to the method disclosed in the literature [Journal of medicinal chemistry, 2013, 56(7): 2959-2974.].
[0042] (2) Monomer M4 was synthesized according to the method disclosed in the literature [Advanced Functional Materials, 2015, 25(23): 3514-3523.].
[0043] (3) Monomers M5, M6, and M7 were synthesized according to the method disclosed in the literature [Journal of the American Chemical Society, 2011, 133(5): 1405-1418.].
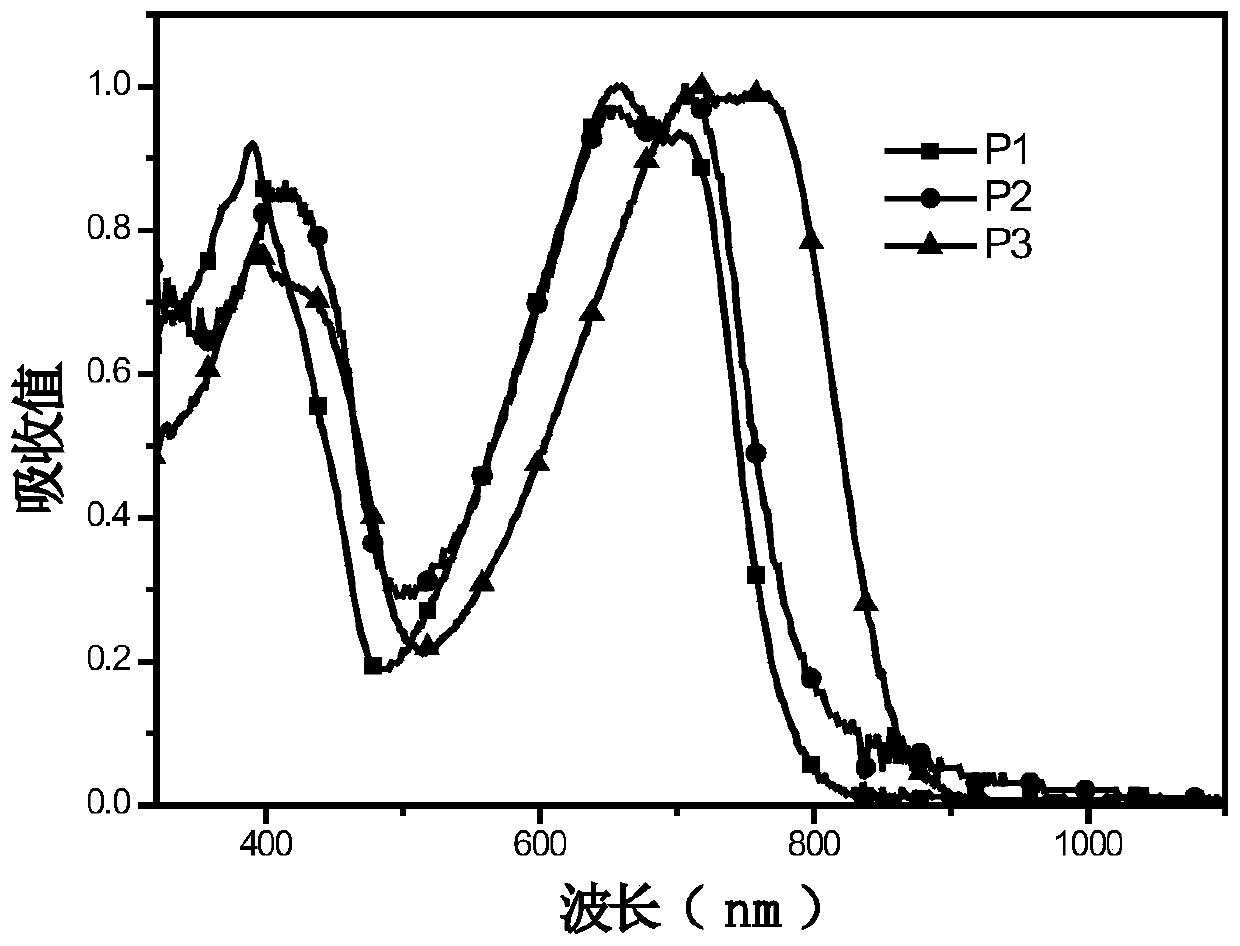
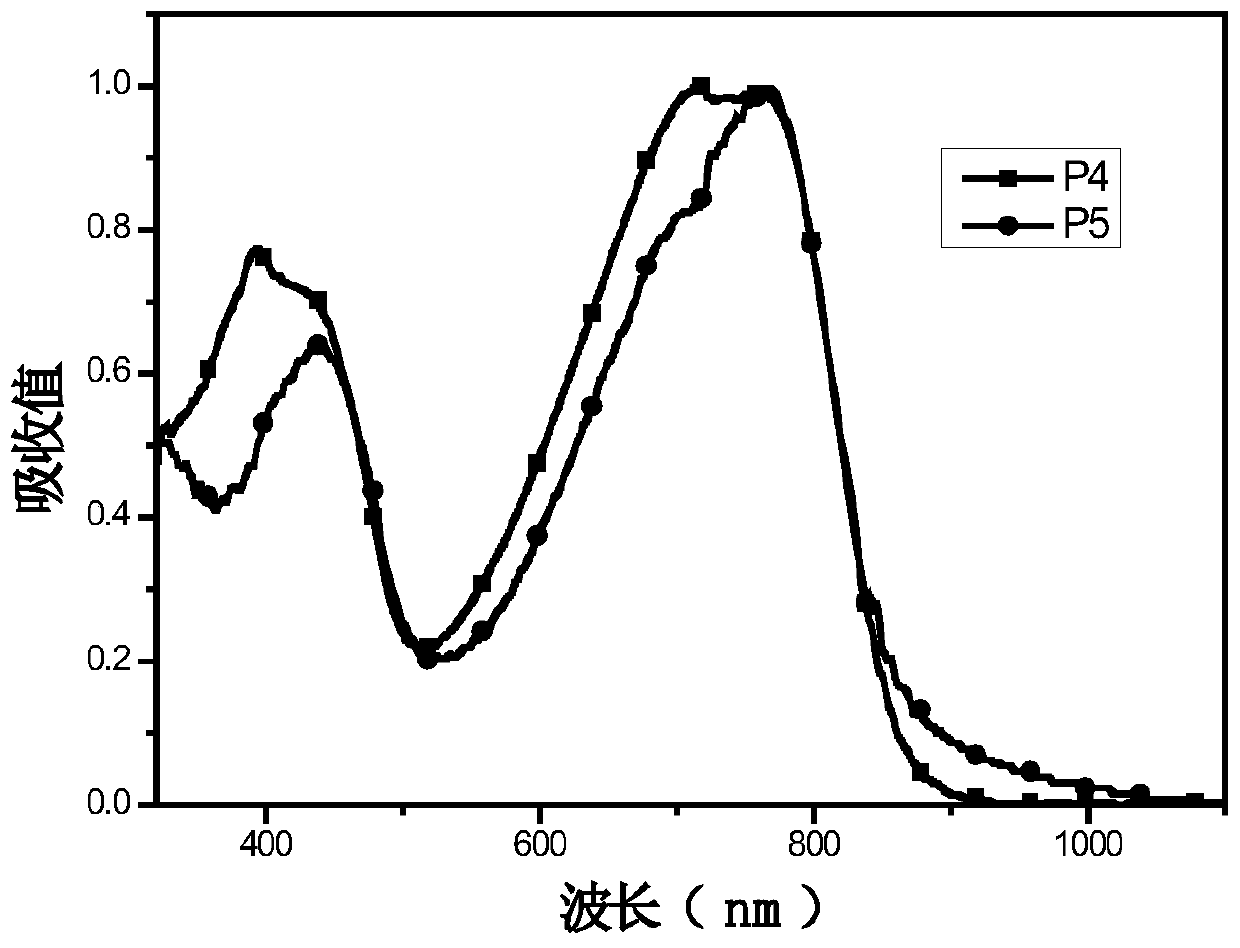
[0044] (4) Synthesis of polymers P1, P2, P3:
[0045]Add monomer M1 (0.4mmol) and monomer M5 (0.5mmol) into a 25mL two-necked flask, pass through nitrogen protection, and add 8mL of toluene. Add 5mg Pd(PPh 3 ) 4 After reacting at 95°C for 1 h, monomer M4 (0.1 mmol) was added, and after continuing the reaction for 12 h, the polymer was precipitated with methanol and washed three times. A dark polymer P1 was obtained with a yield of 80.7%....
Embodiment 2
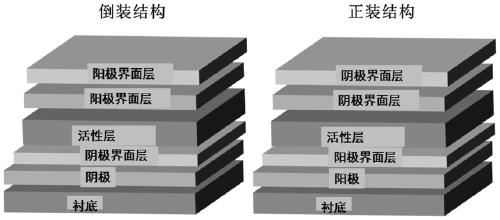
[0053] The conjugated polymers P1, P2, and P3 synthesized in Example 1 are used as electron acceptors in organic photovoltaic devices (ITO cathode / cathode interface layer / active layer / anode-machine interface layer / anode)
[0054] Pre-cut the ITO conductive glass with a square resistance of 20 ohms / cm2 into 15mm×15mm square pieces. Use acetone, special detergent for micron-sized semiconductors, deionized water, and isopropanol to clean ultrasonically in sequence, blow nitrogen whistle, and place in a constant temperature oven for later use. Spin-coat a layer of 5nm thick PFN-Br on ITO, then spin-coat active layer materials PBDB-T / P1, PBDB-T / P2, PBDB-T / P3 with a thickness of 110nm, and finally evaporate MoO 3 and Al electrodes. All preparations were carried out in a glove box under a nitrogen atmosphere. The current-voltage curves of the fabricated flip-chip devices are as follows: Figure 4 The relevant data are listed in Table 1. It can be seen that the main chain structur...
Embodiment 3
[0056] Using the conjugated polymers P1, P2, and P3 synthesized in Example 1 (the AB components are the same in the structure) as electron acceptors in organic photovoltaic devices (ITO anode / anode interface layer / active layer / cathode interface layer / cathode) middle application
[0057] Pre-cut the ITO conductive glass with a square resistance of 20 ohms / cm2 into 15mm×15mm square pieces. Use acetone, special detergent for micron-sized semiconductors, deionized water, and isopropanol to clean ultrasonically in sequence, blow nitrogen whistle, and place in a constant temperature oven for later use. Spin-coat a layer of PEDOT:PSS with a thickness of 20nm on the ITO, and then spin-coat active layer materials PBDB-T / P1, PBDB-T / P2, and PBDB-T / P3 with a thickness of 100nm. Then spin-coat a layer of PFN-Br with a thickness of 5nm, and finally evaporate Al electrodes. All preparations were carried out in a glove box under a nitrogen atmosphere. The current-voltage curves of the prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com