Organic dye, its production and use
An organic dye and reaction technology, applied in the field of organic dye and its preparation, can solve the problems of complex synthesis, troublesome separation and purification, waste of photons, etc., and achieve the effect of simple synthesis and purification, high absorption and utilization rate, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the preparation of organic dye
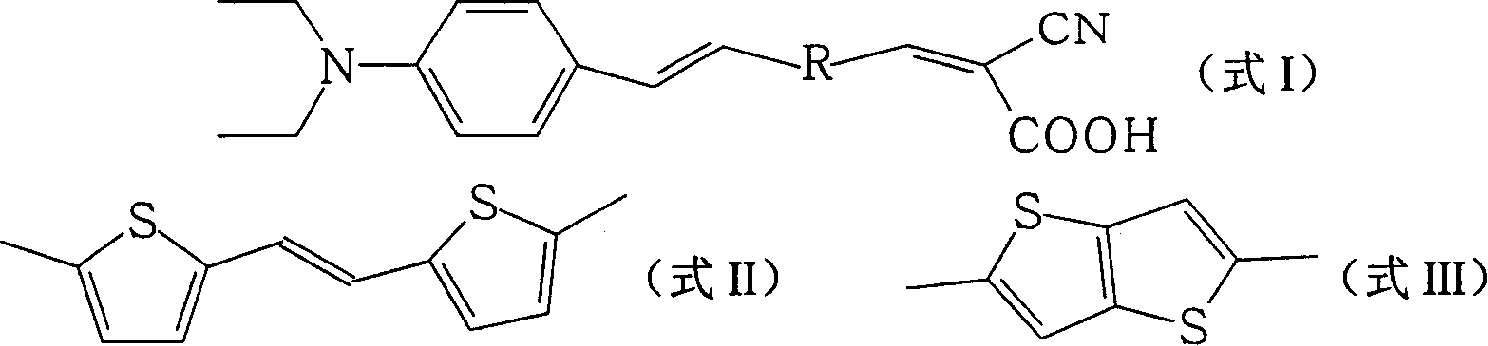
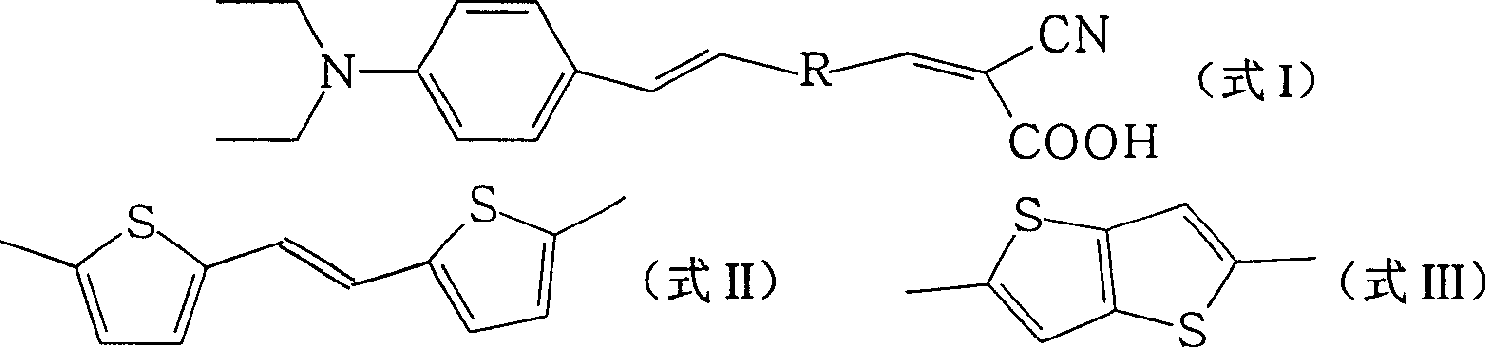
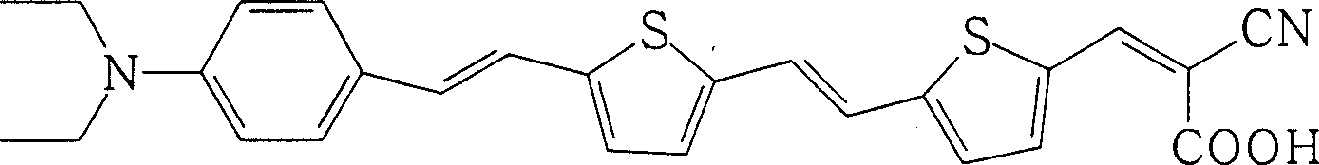
[0023] One, the preparation of organic dye R1 (R in formula I is formula II structure)
[0024]
[0025] 1. Diethylaminobenzyl alcohol quaternary phosphine salt (20.68g) and thiophene-2-carbaldehyde (3.8g) were dispersed in 120ml of anhydrous tetrahydrofuran, and stirred at room temperature. Potassium tert-butoxide (15.5 g) was dissolved in 40 ml of anhydrous tetrahydrofuran, slowly added dropwise to the reaction system at room temperature, and stirred at room temperature for 10 h. Pour 30ml of water into the reaction system, stir for 10min, extract with chloroform, collect the oil phase, and dry over anhydrous sodium sulfate. After the solvent was removed by rotary evaporation, the residue was passed through a silica gel column, and the mobile phase was a mixed solvent of petroleum ether and dichloromethane with a volume ratio of 4:1, and the intermediate product a was eluted.
[0026] 2. Dissolve 20ml of DMF (dimethy...
Embodiment 2
[0038] Embodiment 2, preparation and performance measurement of solar cell device
[0039] The FTO glass was washed with water and ethanol, respectively, O 3 / UV treatment for 18 minutes, and then coated with a layer of nano-TiO 2 Colloid, heated at 450°C for 30 minutes, cooled to room temperature, immersed in 0.5mmol / L dye solution for 12 hours. The electrolyte layer includes 0.6M N-methyl-N-butyl imidazolium salt, 0.05M I 2 , 0.1M LiI, 0.5M tert-butylpyridine, dissolved in acetocyanide and valerocyanide solution with a volume ratio of 1:1, and then form a sandwich structure solar cell device with a platinum counter electrode. Using a 300W xenon lamp as the light source, the measured photoelectric conversion efficiencies of the two dyes are 2.68% (dye R1: R is the structure of formula II) and 2.63% (dye R2: R is the structure of formula III).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com