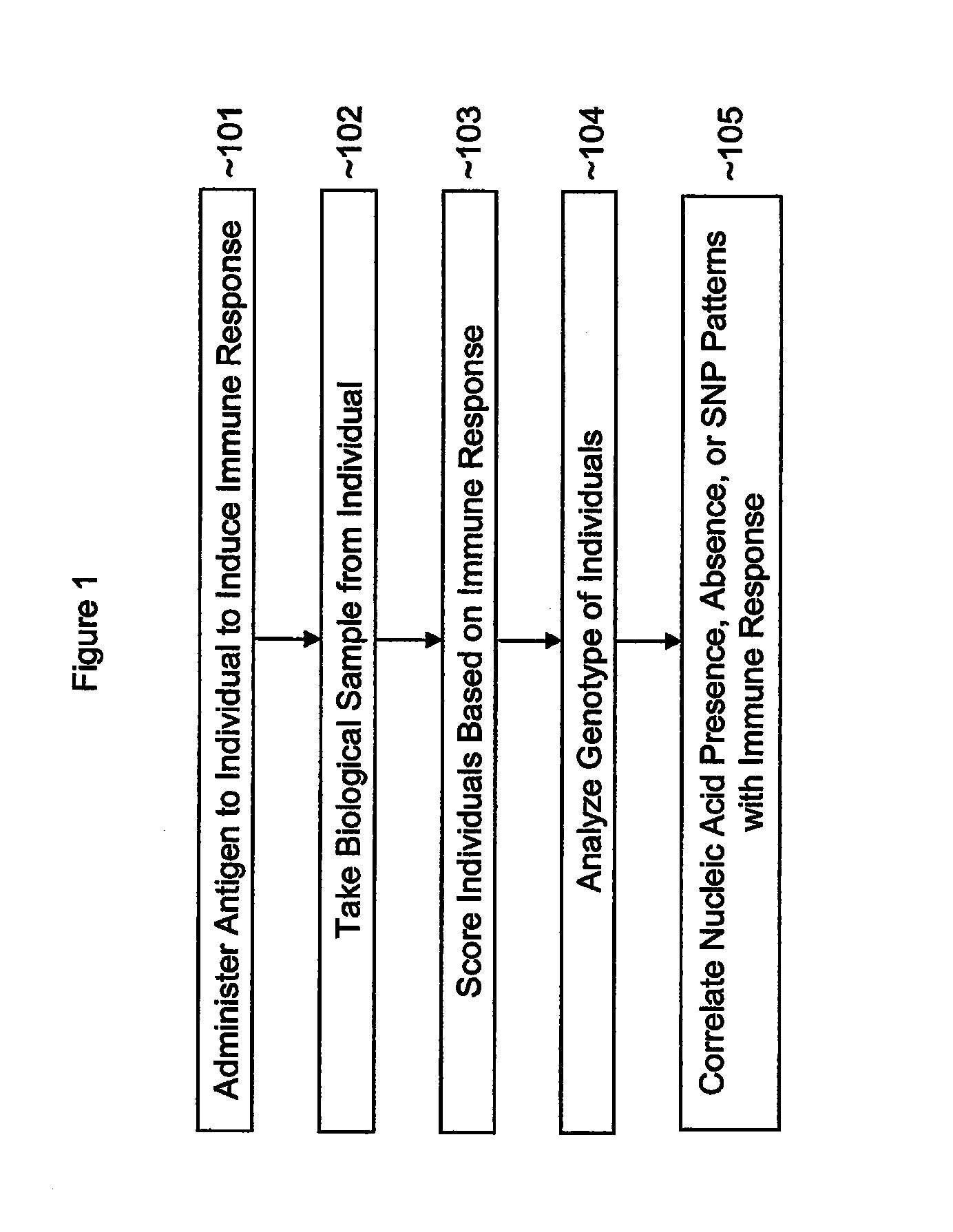
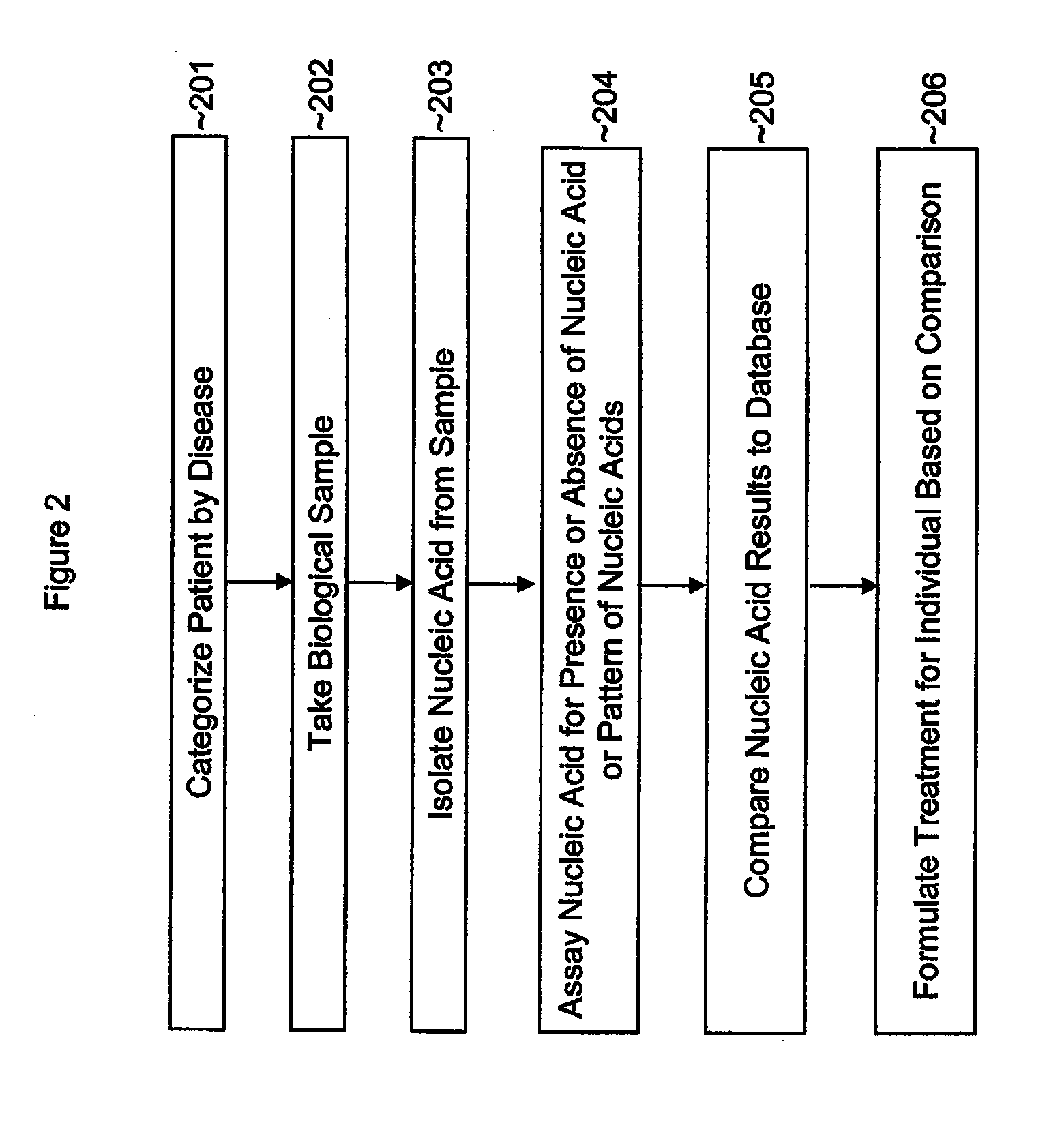
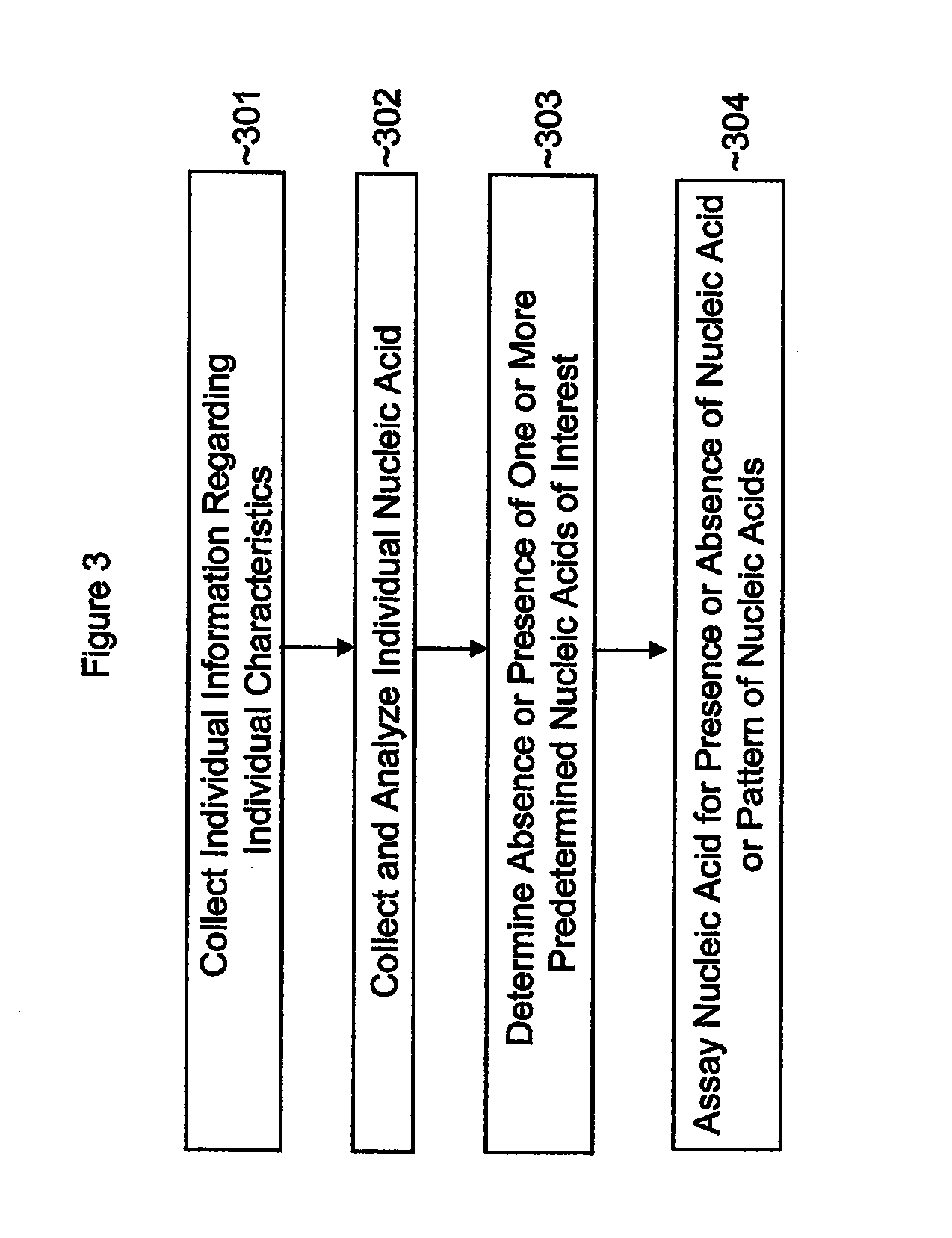
Single nucleotide polymorphisms (SNP) and association with resistance to immune tolerance induction
a single nucleotide polymorphism and immune tolerance technology, applied in the field of single nucleotide polymorphisms and association with resistance to immune tolerance induction, can solve the problems of heterogeneous patient response to treatment with pharmaceuticals, both beneficial and detrimental, and achieve the effect of facilitating tolerance induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Rheumatoid Arthritis (RA) Patients
[0182]120 RA patients on maintenance conventional therapies for their RA were tested for the ability of their peripheral blood mononuclear cells (PBMC) to produce IFNγ when cultured for six days with the autoantigen, purified bovine α1 chain of Collagen II (CII) or α1(II) at 50 μg / u. Culture supernatants were harvested and IFNγ levels determined by ELISA.
IFNγα1(II)S.I.=IFNγα1(II)-IFNγPBSIFNγPBS-100.
As shown below (Table I), 76 patients had increased by two-fold over their unstimulated or (PBS) cultured PBMC, or a prevalence of 63% RA patients with an immune responses to CII (termed “Responders”). Although there is a high prevalence of RA patients with CII autoimmunity, it is one of several possible antigens apparent during the disease.
TABLE IPERCENTAGE of RA PATIENTS WITH IMMUNE RESPONSE byCULTURED PBMC to CII*PatientN%Mean IFNγα1(II) SI ± SEMResponders E76631494 ± 313Non-Responders443720.6 ± 6.5Total120100p = **Mann-Whitney Rank Sum Test
example 2
Low Dose Collagen II Induces Tolerance in DBA / 1 Lac Mice
[0183]Groups of 12 mice were fed oral CII at the doses indicated 8 times over 2 weeks and immunized with 100 μg bovine CII in CFA. After 4 days rest, the mice were then immunized at the base of the tail with 100 μg CII emulsified with complete Freund's adjuvant. The degree of arthritis was assessed by a blinded observer for 8 weeks.
[0184]As can be seen in FIG. 6, 10 μg / day oral dose of CII was most effective in reducing the incidence of arthritis with 500 μg / day being also, but less, effective. The percent of mice with grade 3 or 4 arthritis is indicated on the Y-axis. The biphasis response is due to induction of different tolerance mechanisms by low dose vs high dose CII. Low dose CII (10 μg) induces regulatory T cells while high dose (500 μg) induces anergy or clonal deletion.
example 3
NSAID Inhibition of Induction of Immune Tolerance to Collagen II in DBA / 1 Mice
[0185]To determine whether tolerance induction to orally fed bovine CII in DBA / 1 mice would be abrogated by orally fed NSAID, 3 groups of 20-22 DBA / 1 mice were fed (by gavage) eight doses (Monday, Tuesday, Thursday, and Friday for 2 weeks) of the following: Placebo (saline) in the a.m. and Placebo (0.1 M HAc) in the p.m.; Placebo (saline) in the a.m. and 10 μg native bovine CII in the p.m.; or piroxicam (2.4 μg / gm) in the a.m. and native bovine CII (10 μg) in the p.m. After 1 week, all mice were immunized (intradermally at the base of tail) with 100 μg of bovine CII emulsified in complete Freund's adjuvant. Animals were placed in coded cages and were scored by a blinded observer twice weekly for the number of arthritic joints (joints swollen, red, and / or deformed).
[0186]As shown in FIG. 7, compared to Placebo+Placebo fed controls, the percent of arthritic joints was less over the observation period in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com