Targeted genetic engineering vaccine for African swine fever immune system
An African swine fever, targeted technology, applied in the field of vaccines, can solve problems such as poor immune effect, achieve good immune effect, prevent antibody dependence (ADE) effect, and enhance the effect of cellular immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Obtaining the epitopes of p72 protein, p54 protein and p30 protein
[0027] 1.1 Screen suitable neutralizing epitopes from the amino acid sequences of p72 protein, p54 protein and p30 protein, and then design the amino acid sequence of the African swine fever fusion protein of the present invention.
[0028] The amino acid sequence of p72 protein is: MASGGAFCLIANDGKADKIILAQDLLNSRISNIKNVNKSYGKPDPEPTLSQIEETHLVHFNAHFKPYVPVGFEYNKVRPHTGTPTLGNKLTFGIPQYGDFFHDMVGHHILGACHSSWQDAPIQGTSQMGAHGQLQTFPRNGYDWDNQTPLEGAVYTLVDPFGRPIVPGTKNAYRNLVYYCEYPGERLYENVRFDVNGNSLDEYSSDVTTLVRKFCIPGDKMTGYKHLVGQEVSVEGTSGPLLCNIHDLHKPHQSKPILTDENDTQRTCSHTNPKFLSQHFPENSHNIQTAGKQDITPITDATYLDIRRNVHYSCNGPQTPKYYQPPLALWIKLRFWFNENVNLAIPSVSIPFGERFITIKLASQKDLVNEFPGLFVRQSRFIAGRPS RRNIRFKPWFIPGVINEISLTNNELYINNLFVTPEIHNL FVKRVRFSLIRVHKTQ VTHTNNNHHDEKLMSALKWPIEYMFIGLKPTWNISDQNPHQHRDWHKFGHVVNAIMQPTHHAEISFQDRDTALPDACSSISDISPVTYPITLPIIKNISVTAHGINLIDKFPSKFCSSYIPFHYGGNAIKTPDDPGAMVSAAINMITFALKPREEYQPSGHINDYVSASLLMITFALKPREE...
Embodiment 2
[0074] Example 2 Prokaryotic expression system was used to express and purify the fusion protein according to the amino acid sequence of the designed fusion protein
[0075] (1) Construction of a prokaryotic expression plasmid: the amino acid sequence of the African swine fever fusion protein of the present invention was handed over to Shenggong Bioengineering (Shanghai) Co., Ltd., and the company synthesized the corresponding code for the above-mentioned African pig according to the provided amino acid sequence The nucleotide sequence of the plague fusion protein, and clone the synthesized nucleotide into a suitable plasmid vector.
[0076] (2) Expression of African swine fever fusion protein: The synthetic plasmid containing the African swine fever fusion protein was transferred into BL21(DE3), and the expression engineering bacteria were constructed, and inoculated to 100μg / mL Kan at an inoculum of 1%. (Kanamycin) 100mL LB liquid culture medium, 37℃, 200r / min, when the A600 is 0...
Embodiment 3
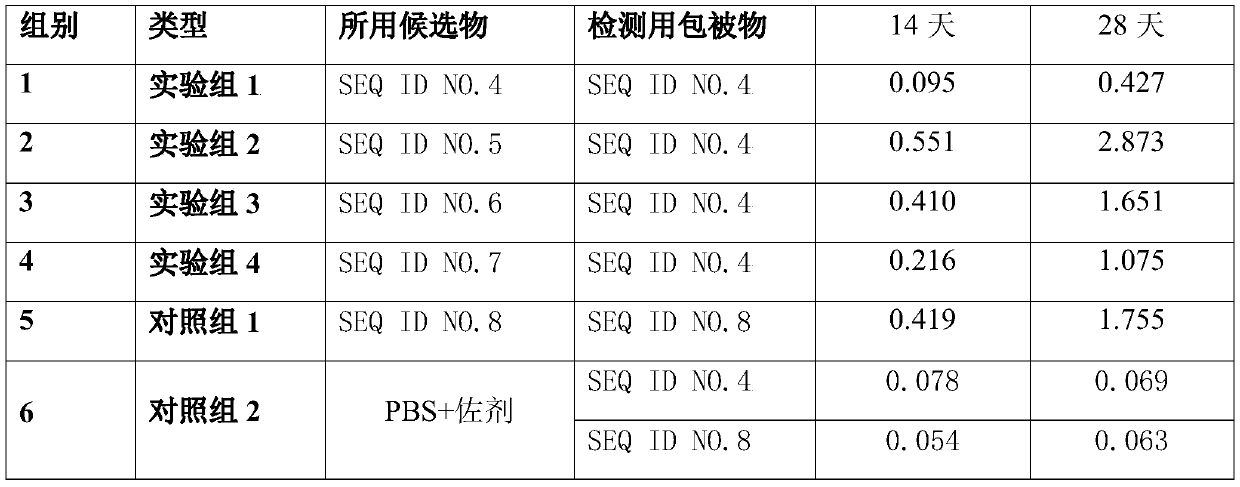
[0079] 3.1 Preparation of African swine fever vaccine: take the African swine fever fusion protein solution prepared in Example 2 and dilute it to 0.2 mg / ml, mix it with adjuvant 201 (Sebik, France) in equal volume, and emulsify it with an emulsifier for 25 minutes.
[0080] 3.2 Verify the immune effect of the prepared African swine fever vaccine
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com