Novel coronavirus nucleic acid detection kit, preparation method and application
A detection kit and virus nucleic acid technology, applied in the field of RNA in vitro amplification, can solve problems such as false positives, irregular sampling techniques and methods, and failure to obtain viruses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
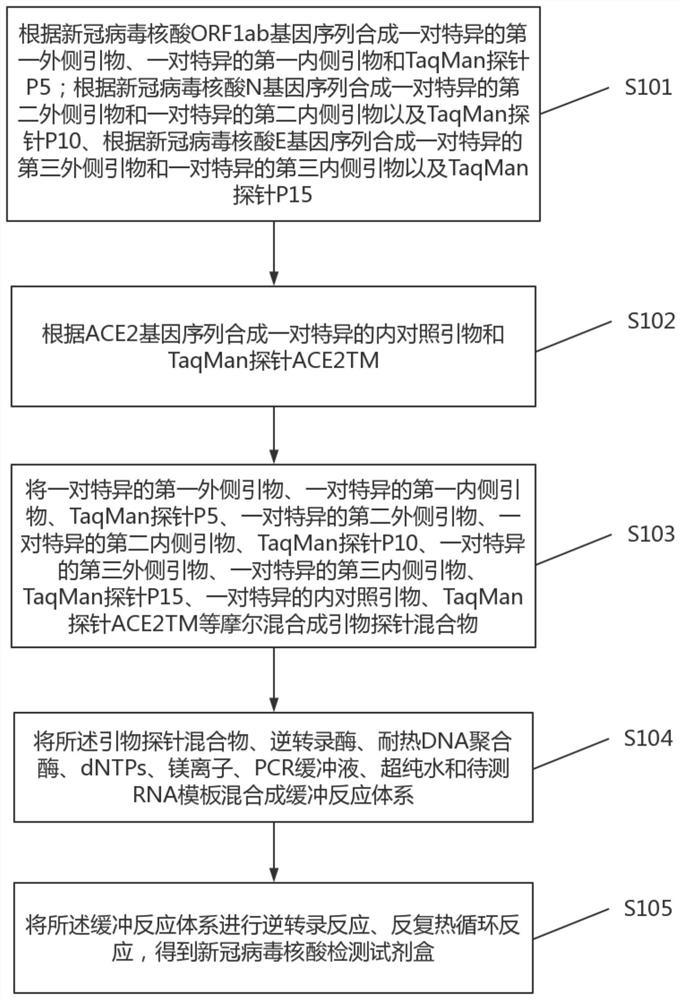
[0073] Second, see figure 1 , figure 1 It is a schematic flow chart of a preparation method of a novel coronavirus nucleic acid detection kit provided by an embodiment of the present invention. Specifically, the preparation method of the novel coronavirus nucleic acid detection kit may include the following steps:
[0074] S101. Synthesize a pair of specific first outer primers, a pair of specific first inner primers and TaqMan probe P5 according to the sequence of the new coronavirus nucleic acid ORF1ab gene; synthesize a pair of specific second outer primers and A pair of specific second inner primers and TaqMan probe P10, synthesize a pair of specific third outer primers and a pair of specific third inner primers and TaqMan probe P15 according to the sequence of the new coronavirus nucleic acid E gene;
[0075] S102. Synthesizing a pair of specific internal control primers and TaqMan probe ACE2TM according to the ACE2 gene sequence;
[0076] S103. Combine a pair of specif...
Embodiment 1
[0081] Example 1, using the virus RNA extraction kit to extract sputum sample RNA as a template to be amplified; design primer probes: synthesize a pair of specific outer primers, a pair of specific inner primers and TaqMan Probe P5, synthesize a pair of specific second outer primers, a pair of specific second inner primers and TaqMan probe P10 according to the sequence of the new coronavirus nucleic acid N gene, and synthesize a pair of specific third outer primers based on the sequence of the new coronavirus nucleic acid E gene Primers, a pair of specific third internal primers and TaqMan probe P15, synthesize a pair of specific internal control primers and TaqMan probe ACE2TM according to the ACE2 gene sequence; reverse transcription NCA-QPCR reaction: RT-NCA-PCR: mixed 50ul reaction System, containing 10 pmol each of inner and outer primers (P1, P2, P3, P4, P5, P6, P7, P8, P9, P10, P11, P12, P13, P14, P15, ACE2F, ACE2R, ACE2TM), template RNA 1-5ng, 1X PCR buffer, RNA polym...
Embodiment 2
[0082] Example 2, using the conventional phenol-chloroform extraction method to extract peripheral blood DNA as a template to be amplified; design primer probes: synthesize a pair of specific outer primers and a pair of specific inner primers according to the sequence of the new coronavirus nucleic acid ORF1ab gene and TaqMan probe P5, synthesize a pair of specific second outer primers, a pair of specific second inner primers and TaqMan probe P10 according to the N gene sequence of the new coronavirus nucleic acid, and synthesize a pair of specific first primers based on the N gene sequence of the new coronavirus nucleic acid. Three outer primers, a pair of specific third inner primers and TaqMan probe P15, synthesize a pair of specific internal control primers and TaqMan probe ACE2TM according to the ACE2 gene sequence; reverse transcription NCA-QPCR reaction: RT-NCA-PCR: mixed 50ul reaction system, containing 10 pmol each of inner and outer primers (P1, P2, P3, P4, P5, P6, P7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com