Detection reagent kit of fluorescent quantitation PCR latent hepatitis b viruses
A fluorescent quantitative and hepatitis B virus technology, applied in the direction of microbial measurement/inspection, DNA/RNA fragments, recombinant DNA technology, etc., can solve the problems of HBV base mutation, OBI missed detection, low practicability, etc., and achieve strong specificity , High detection efficiency, efficient and accurate quantitative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Sensitivity test of quantitative detection of HBV by fluorescence quantitative PCR of single-plex S gene region
[0115] 1. Dissolve the standard positive template with distilled water and dilute it to 3×10 5 copies / μL, 3×10 4 copies / μL, 3×10 3 copies / μL, 3×10 2 copies / μL, 3×10 1 copies / μL, 3×10 0 copies / μL.
[0116] 2. Fluorescent quantitative PCR experiment (20 μL amplification reaction system): 10.0 μL fluorescent quantitative reaction solution, 0.7 μL (0.5 μM) forward primer of the S gene region primer set, 0.7 μL (0.5 μM) reverse primer of the S gene region primer set , fluorescent probe 1.5 μL (0.2 μM), primer probe set selected from combination S1, standard positive template 7.0 μL.
[0117] Fluorescent quantitative PCR reaction parameters are as follows: pre-denaturation: temperature is 95°C, time is 10min; denaturation: temperature is 95°C, time is 10s, annealing: temperature is 62°C, time is 30s, 45 cycles of denaturation and annealing, this step consist...
Embodiment 2
[0119] Sensitivity test of quantitative detection of HBV by fluorescence quantitative PCR of single C gene region
[0120] Fluorescent quantitative PCR experiment (20 μL amplification reaction system): 10.0 μL fluorescent quantitative reaction solution, 0.7 μL (0.5 μM) forward primer, 0.7 μL (0.5 μM) reverse primer of C gene region primer set, 1.5 μL fluorescent probe ( 0.2 μM), the primer probe set was selected from combination C1, and the standard positive template was 7.0 μL.
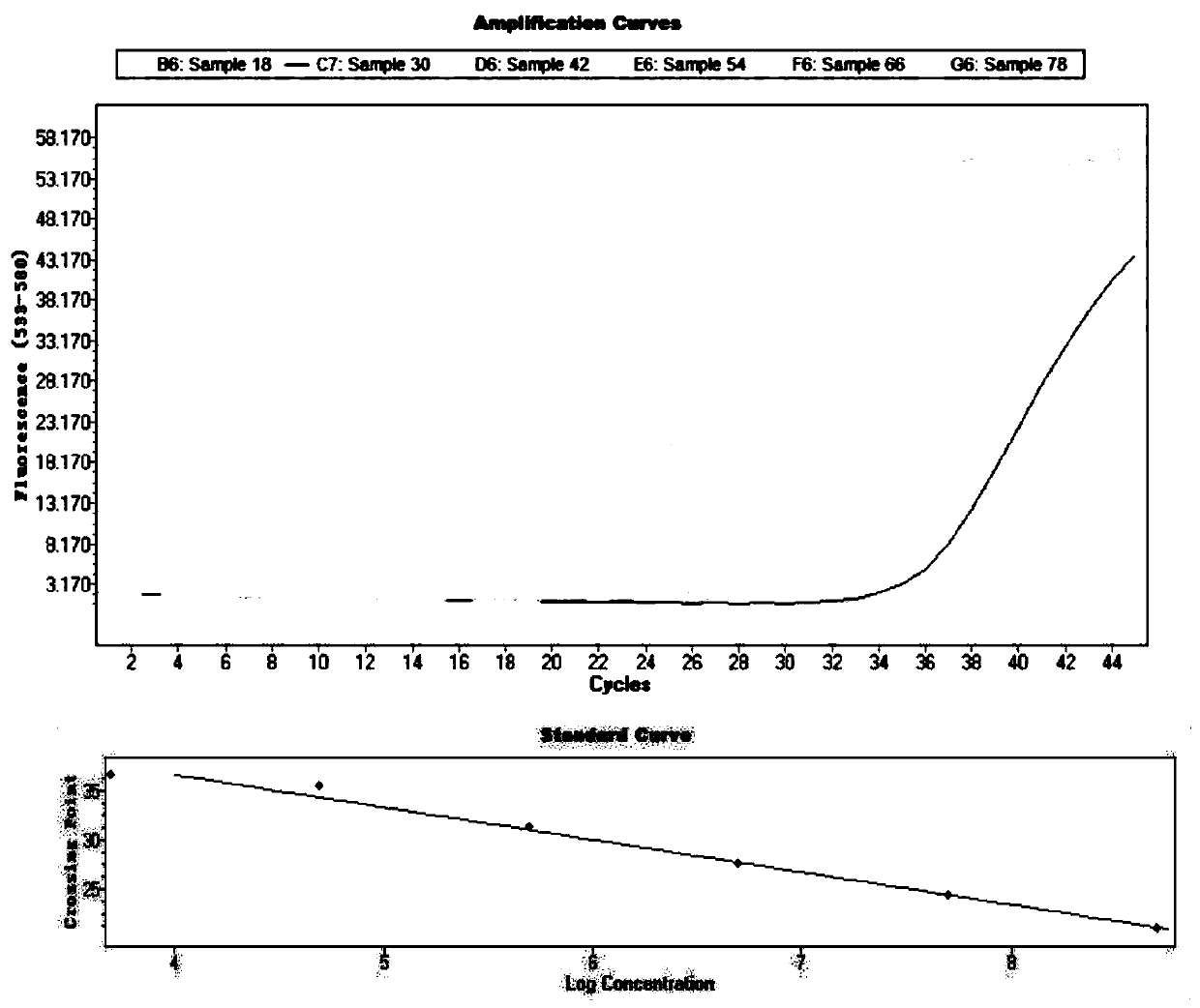
[0121] Fluorescent quantitative PCR reaction parameters are as in Example 1, and the results are shown in the attached figure 2 .
[0122] The results showed that the minimum detection concentration of the S and C gene regions of HBV plasmid DNA by single-plex fluorescent quantitative PCR was 3.0copies / uL.
Embodiment 3
[0124] Quantitative detection of HBV by double-gene simultaneous fluorescent quantitative PCR
[0125] Fluorescent quantitative PCR experiment (20 μL amplification reaction system): 10.0 μL fluorescent quantitative reaction solution, 0.25 μL (0.5 μM) forward primer of the S gene region primer set, 0.25 μL (0.5 μM) reverse primer of the S gene region primer set, fluorescent Probe 0.50 μL (0.2 μM), C gene region primer set forward primer 0.25 μL (0.5 μM), C gene region primer set reverse primer 0.25 μL (0.5 μM), fluorescent probe 0.50 μL (0.2 μM), primer The probe sets were selected from combinations S1 and C1 respectively, and the standard positive template was 8.0 μL.
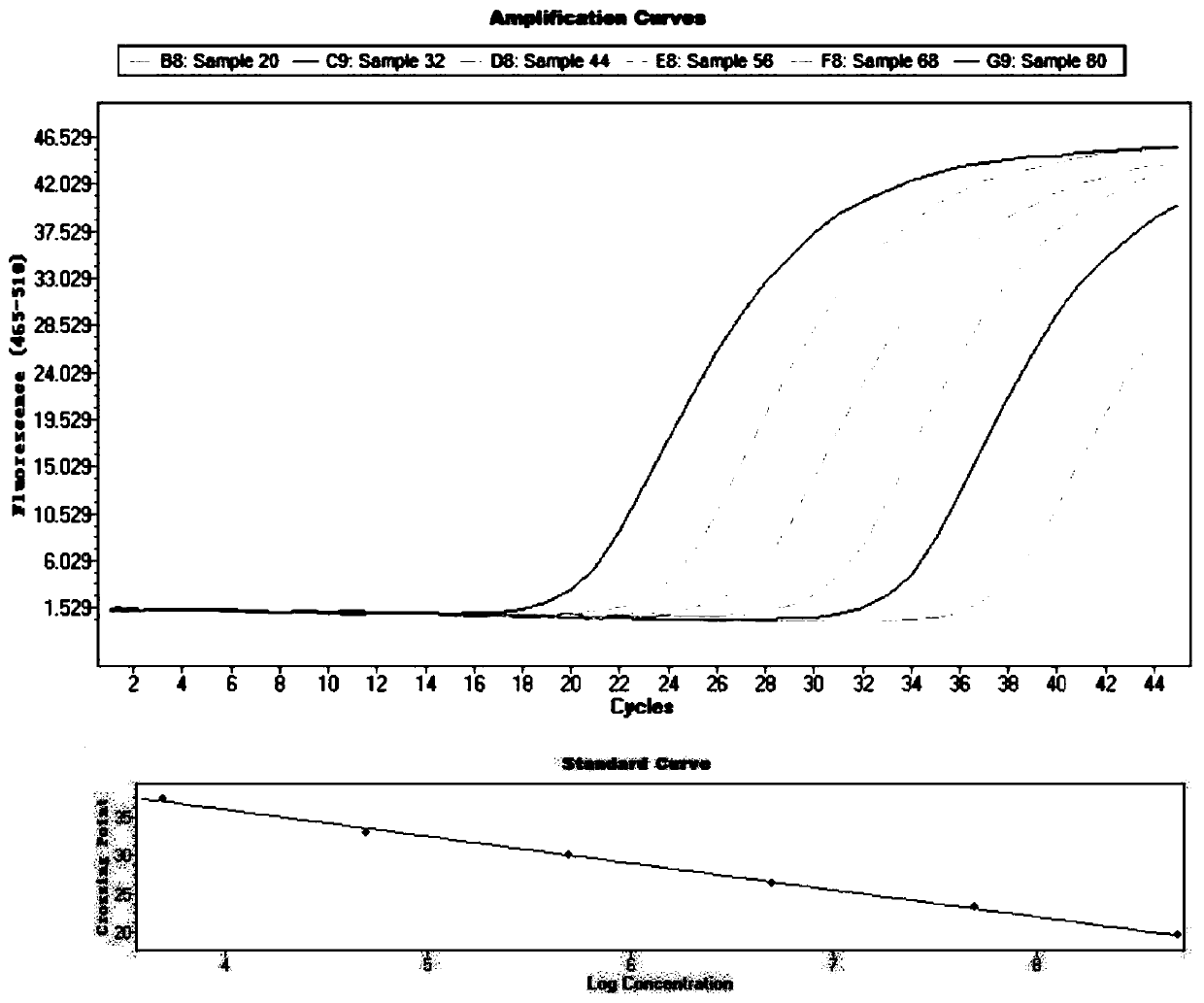
[0126] Fluorescent quantitative PCR reaction parameters are as in Example 1, dual-channel fluorescence simultaneous detection, set as attached image 3 .
[0127] The results of the amplification of the S gene region are shown in the appendix Figure 4 , the results of the amplification of the C gene region ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com