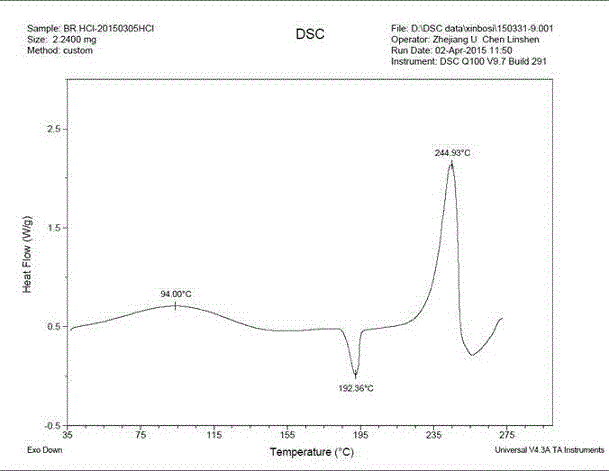
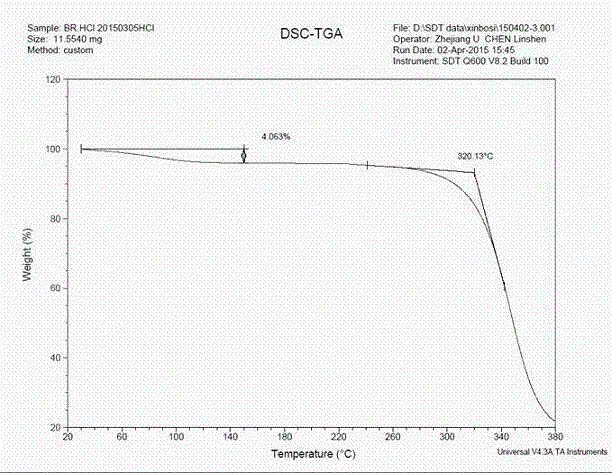
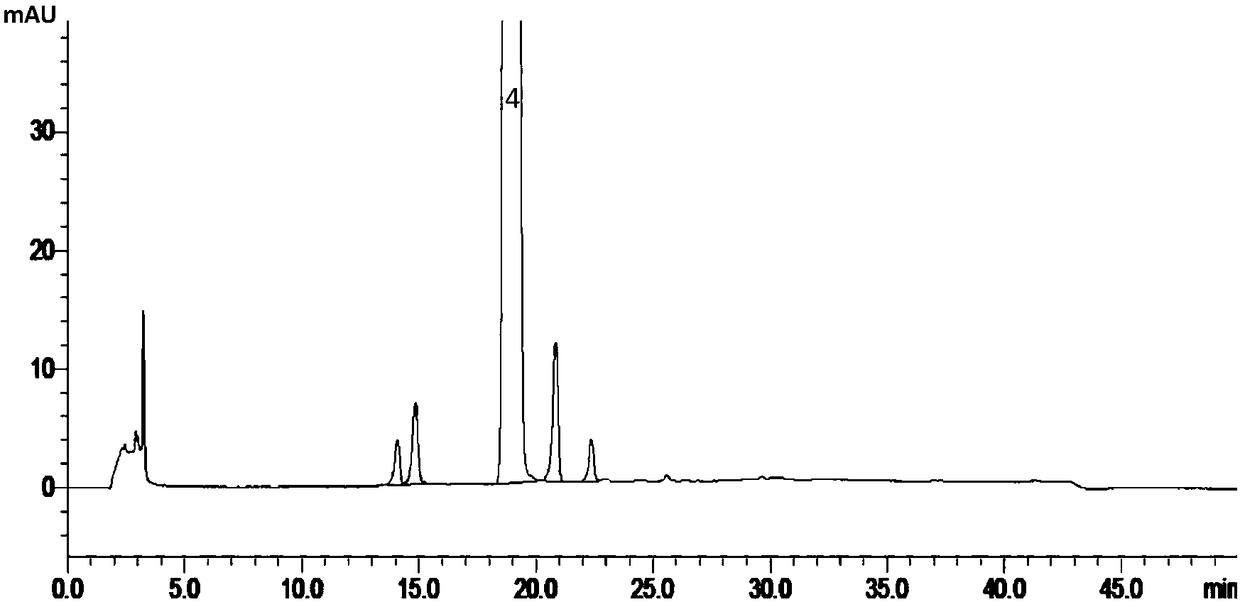
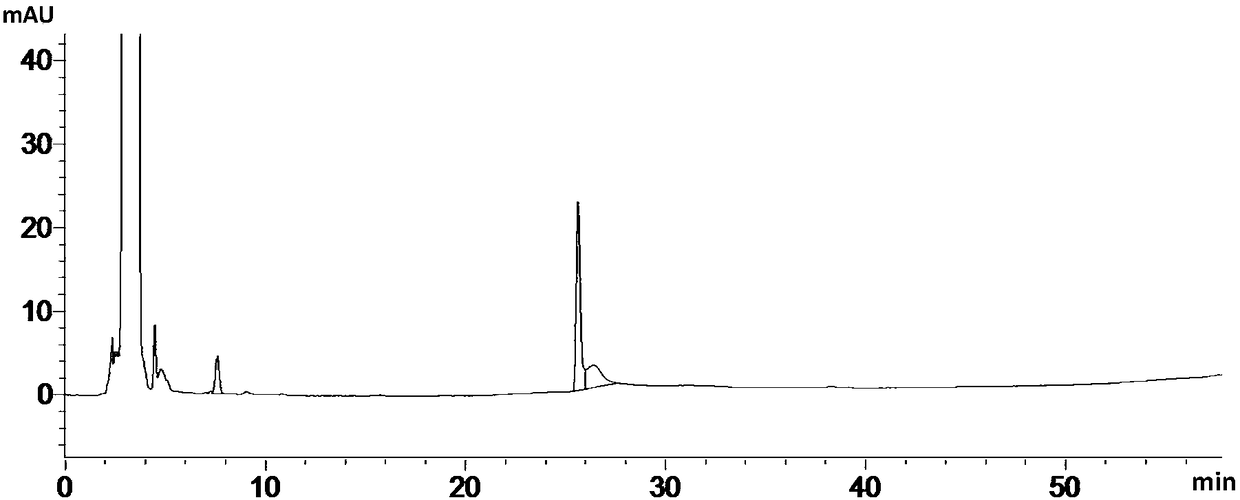
Patents
Literature
72 results about "Brexpiprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
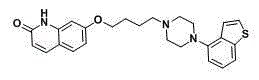
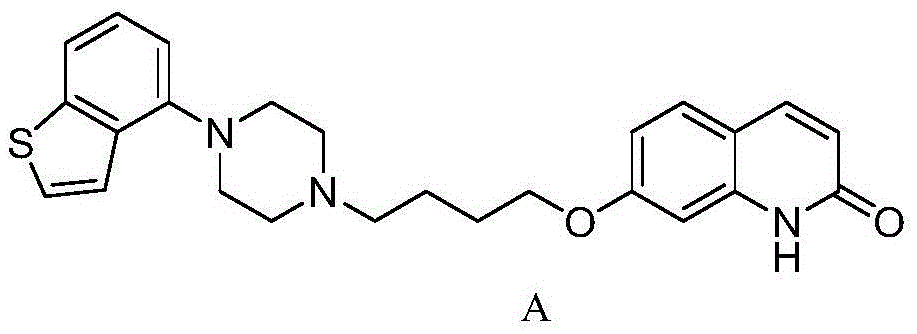
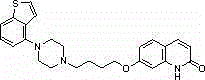
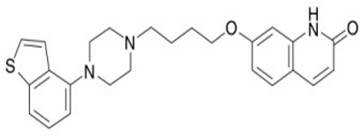
Brexpiprazole, sold under the brand name Rexulti, is an atypical antipsychotic. It is a dopamine D₂ receptor partial agonist and has been described as a "serotonin–dopamine activity modulator" (SDAM). The drug received FDA approval on July 13, 2015 for the treatment of schizophrenia, and as an adjunctive treatment for depression. It has been designed to provide improved efficacy and tolerability (e.g., less akathisia, restlessness and/or insomnia) over established adjunctive treatments for major depressive disorder (MDD).
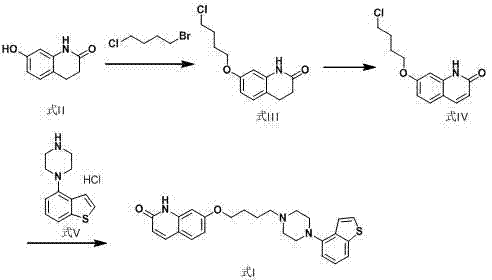
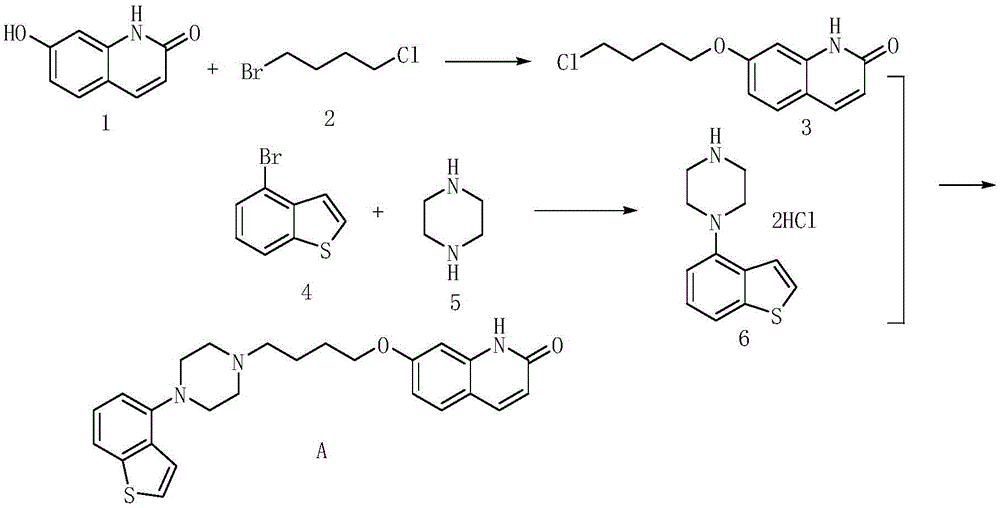
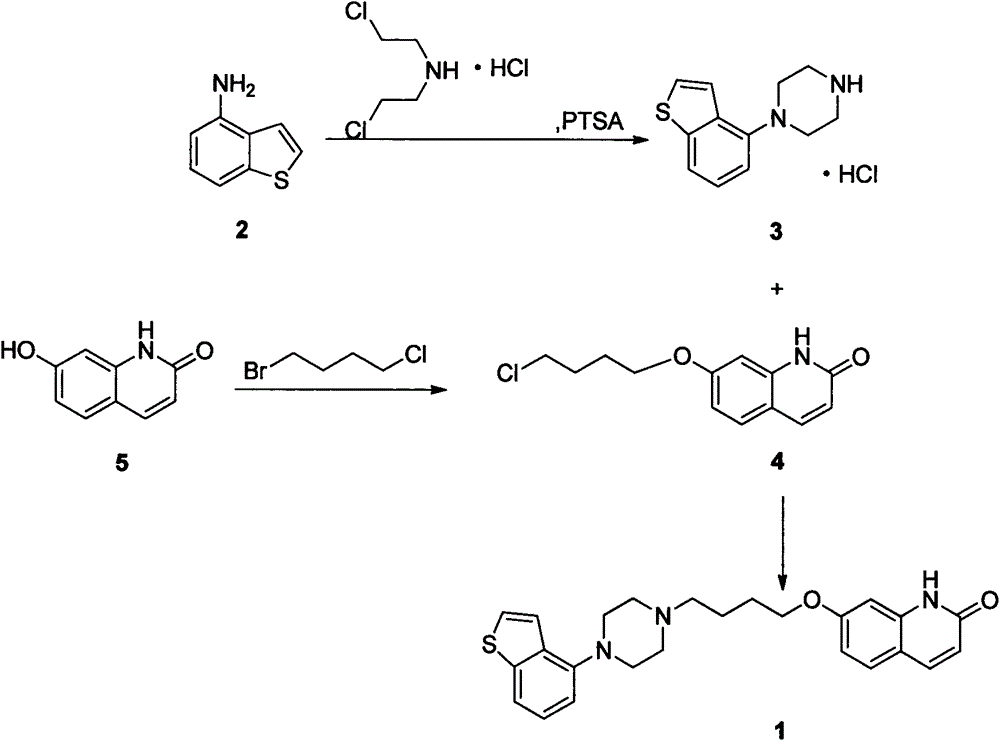
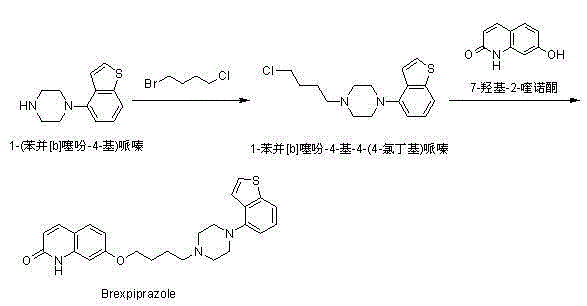
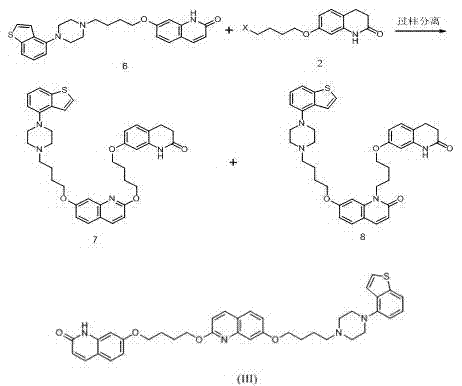
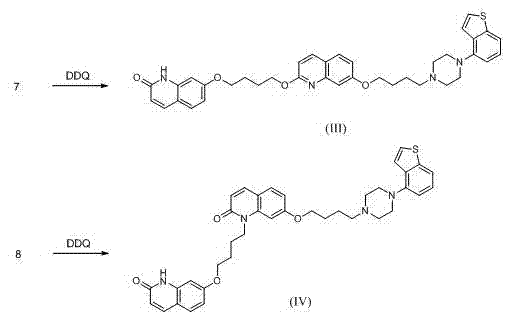
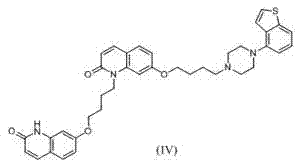
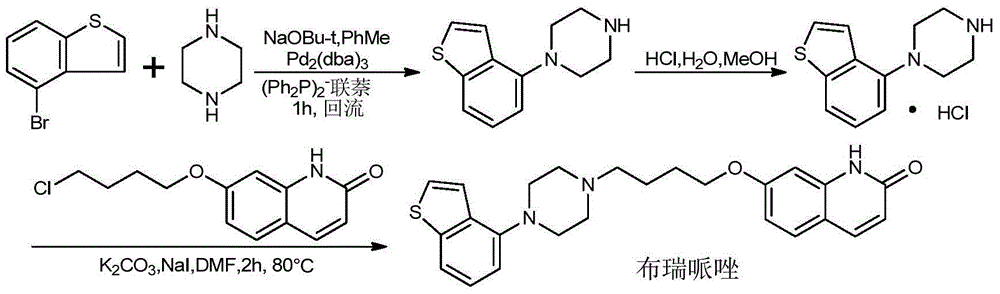
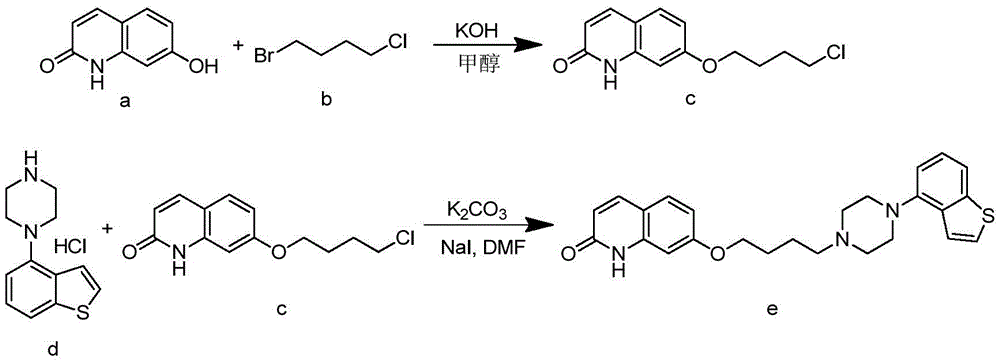
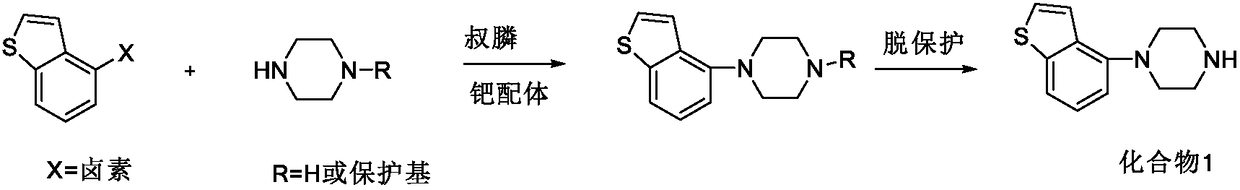
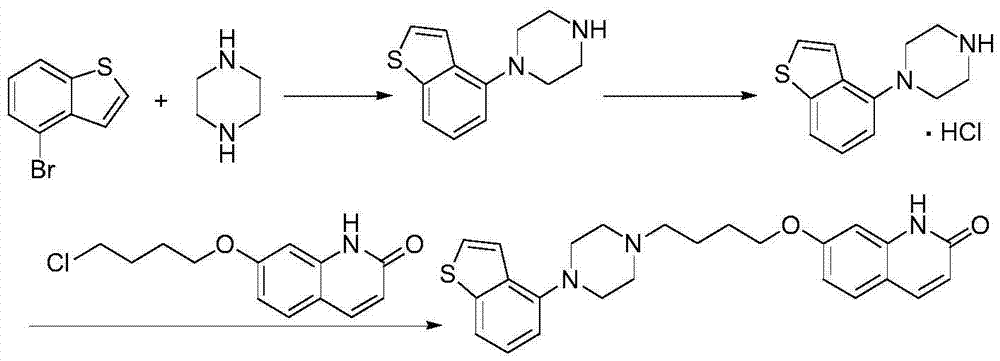
Preparation method of brexpiprazole
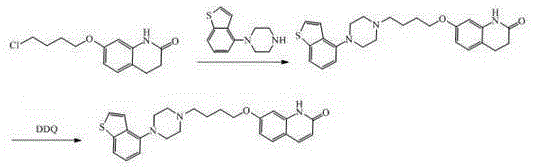
The invention relates to a preparation method of brexpiprazole and an intermediate thereof; the method includes the steps of carrying out a reaction of a compound represented by the formula II with 1-bromo-4-chlorobutane in a reaction solvent to obtain a compound represented by the formula III, in the presence of 2,3-dicyano-5,6-dichlorobenzoquinone, oxidizing the compound represented by the formula III into a compound represented by the formula IV, carrying out a reaction of the compound represented by the formula IV with a compound represented by the formula V to obtain brexpiprazole, then carrying out hydrochloride formation and refining, adding an alkali, and allowing brexpiprazole to drift away. The purity of brexpiprazole obtained by the method is more than 99.5%, the total yield is more than 80%, the process is simple and the cost is low.
Owner:CHONGQING PHARMA RES INST
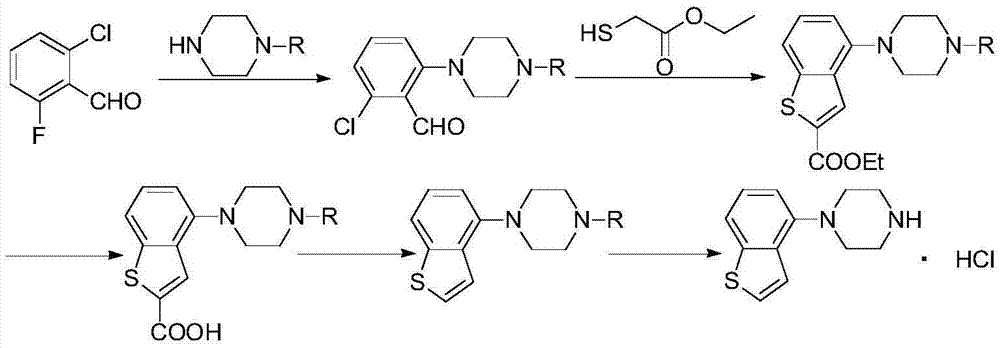
Brexpiprazole preparation method
The present invention relates to a brexpiprazole preparation method, which comprises: (1) carrying out a condensation reaction of a compound represented by a formula III and a compound represented by a formula II in a solvent to prepare a compound represented by a formula IV; and (2) in a solvent, carrying out dehydrogenation on the compound represented by the formula IV with dichloro dicyano benzoquinone to obtain the brexpiprazole. With the method of the present invention, the reaction selectivity can be improved, the impurity generation can be reduced, the low yield problem caused by poor solubility of the key intermediate is avoided, and the total yield of the reaction is higher than 70.0%.
Owner:CHONGQING PHARMA RES INST
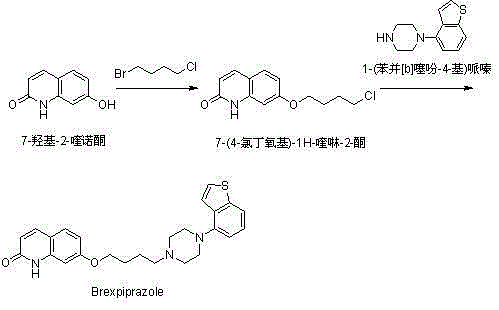
Novel preparation method of brexpiprazole
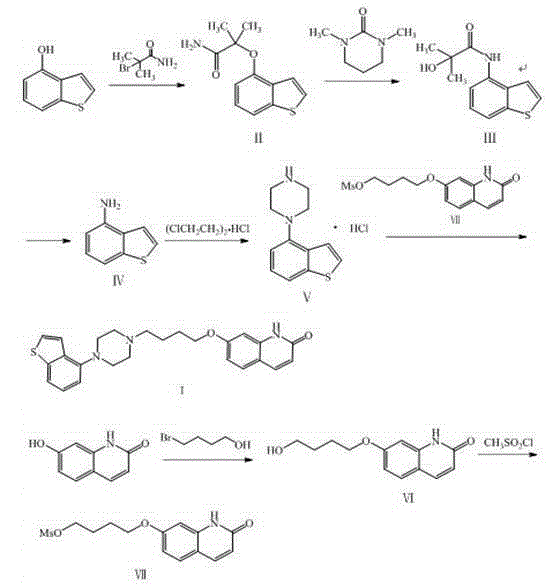
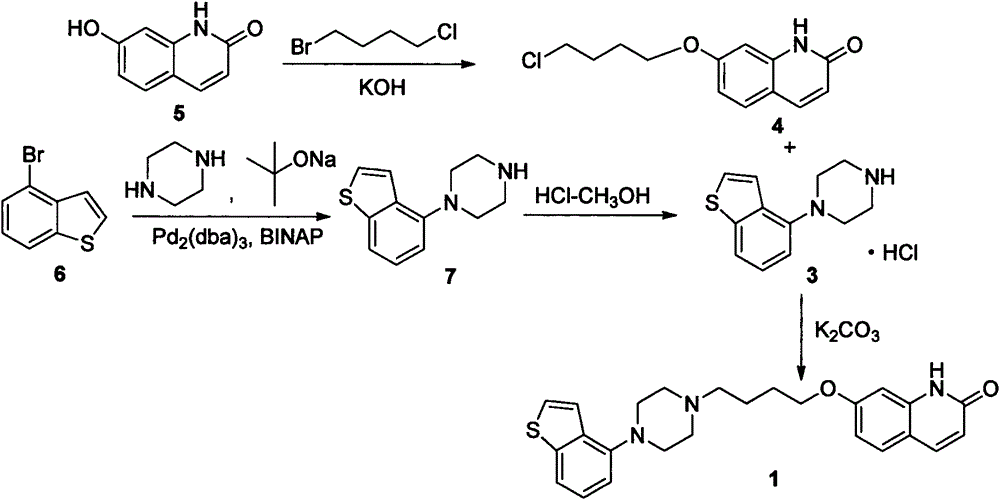
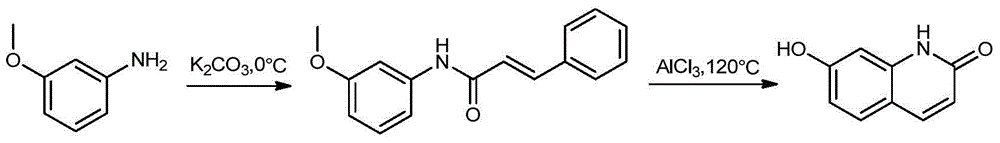
The invention provides a novel preparation method of brexpiprazole, belonging to the technical field of medicines and chemical synthesis, and solving the problems of more impurities, low yield and serious pollution of existing brexpiprazole preparation methods. According to the method, 4-hydroxy benzothiophene is taken as a starting material, an intermediate V is obtained by four steps of reactions, an intermediate VII is obtained from 7-hydroxy-2-quinolone by two steps of reactions, and the intermediate V and the intermediate VII are subjected to condensation to obtain the brexpiprazole meeting clinic medicinal requirements. The preparation method is easy for getting raw materials, low in price, simple in operation, and mild in reaction condition, and has good industrialized application values.
Owner:ANHUI HEALSTAR PHARM CO LTD
Preparing method for brexpiprazole
The invention belongs to the technical field of chemical drug synthesis and particularly relates to a preparing method for brexpiprazole. According to the preparing method, crystalline brexpiprazole is prepared through five steps. The preparing method has the advantages that the synthetic yield is high, operation is simple, purification is easy, and the end product is stable. In addition, a metal catalyst is effectively avoided, and the number of reaction steps is greatly reduced, so that the reaction difficulty is lowered, post-treatment is simplified, the yield is increased, the reaction cost is greatly reduced, and thus industrialized mass production is facilitated.
Owner:NANJING CORE TECH CO LTD
Preparation method of brexpiprazole
The invention discloses a preparation method of a compound brexpiprazole represented by formula (1). The preparation method adopts 4-aminobenzo[b]thiophene as an initial raw material to synthesize a piperazine ring, avoids a heavy metal palladium catalyzed reaction, reduces the synthesis steps and impurities, and reduces the cost.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
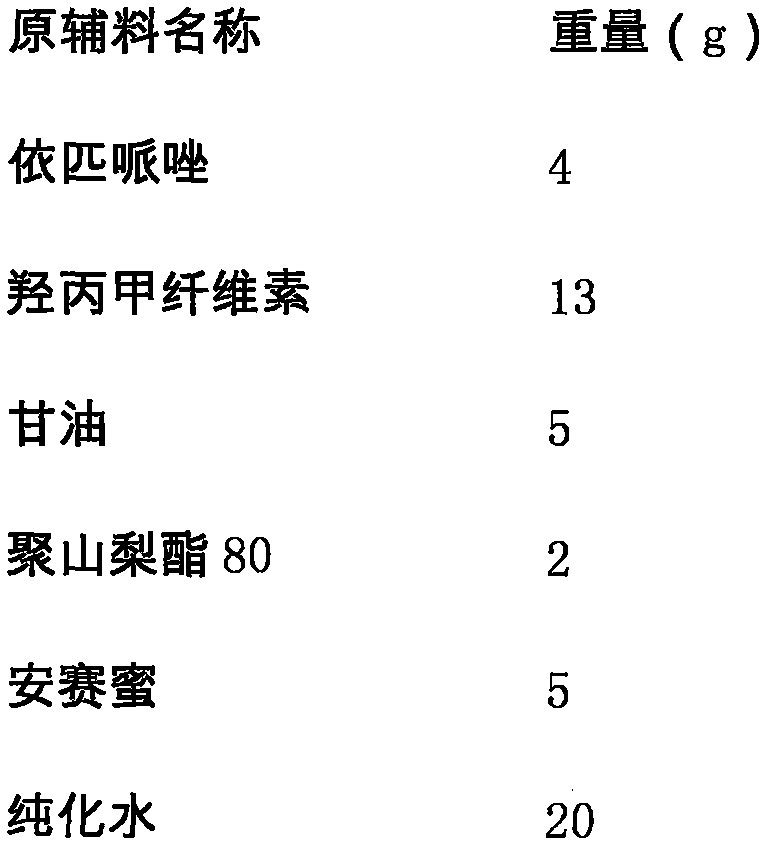
Brexpiprazole oral fast dissolving film
InactiveCN105395528AImprove complianceImprove bioavailabilityOrganic active ingredientsNervous disorderPharmacyPlasticizer
The invention relates to a brexpiprazole oral fast dissolving film which can be dissolved instantly in the oral cavity and is used for improving a using property of brexpiprazole, and belongs to the field of medicinal preparations. The brexpiprazole oral fast dissolving film includes a medicine active ingredient and auxiliary materials applicable to pharmacy. Brexpiprazole is adopted as the medicine active ingredient. The auxiliary materials applicable to the pharmacy include a film forming material, plasticizer, an absorption enhancer, a flavoring agent and the like. The brexpiprazole oral fast dissolving film has the advantages of being large in drug-loading capacity, small in thickness, good in mouthfeel, capable of being dissolved in the oral cavity instantly without drinking water, and high in oral absorption speed, solves the problems that severe depression patients and schizophrenia patients are poor in medicine taking compliance, hide medicine and vomit the medicine, and is particularly suitable for the patients with dysphagia.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Method for preparing Brexpiprazole with one-pot process
ActiveCN105061414ASolve the problem of insufficient reaction and difficult purificationEasy to operateOrganic chemistryBenzeneAlcohol
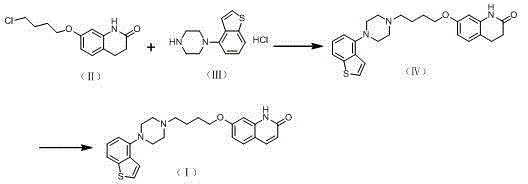
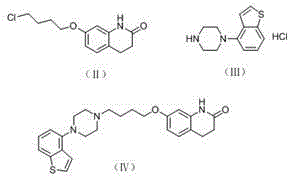
The invention relates to a method for preparing Brexpiprazole with a one-pot process. 7-hydroxyl-2-quinolone reacts with added 1-bromine-4-chlorobutane in the presence of alcohol and alkali, 1-(benzo[B]thiophen-4-yl) piperazine hydrochloride and water are added for a further reaction, finally, filtration, separation and drying are performed, and Brexpiprazole is obtained. Compared with the prior art, the method has the benefits as follows: 1, the problems of insufficient reaction and difficulty in purification in the prior art are solved; 2, the operation process is simplified, and the production efficiency is greatly improved; 3, used solvents are safe, and less environment pollution is caused.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Brexpiprazole orally disintegrating tablets
InactiveCN105412036AGreat tastePromote absorptionOrganic active ingredientsNervous disorderAdditive ingredientOrally disintegrating tablet
The invention discloses brexpiprazole orally disintegrating tablets and a preparation method of the brexpiprazole orally disintegrating tablets. Bexpiprazole serves as a medicine activity component, and powder co-grinding is conducted on brexpiprazole and lactose so that the dissolution of brexpiprazole and lactose can be improved; eudragit serves as a bitter taste masking agent, a co-ground object is mixed with other auxiliaries to form granules, and finally the tablets are formed through pressing. Due to the fact that brexpiprazole is almost undissolved in water, the dissolution of brexpiprazole can be promoted by preparing the orally disintegrating tablets, and bioavailability is improved; eudragit is used for masking the bitter taste, the bitter and numb taste of brexpiprazole is effectively masked, and the medicine taking compliance of a patient is enhanced.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
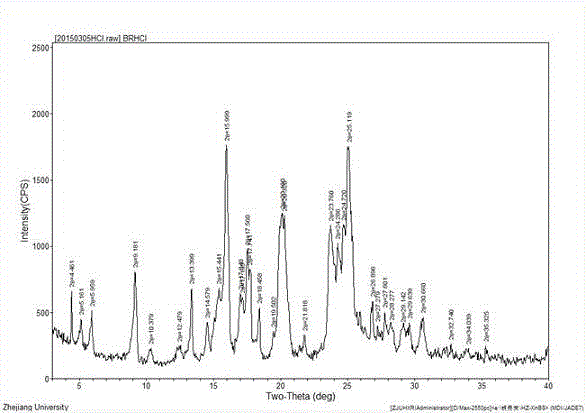
Crystal form A brexpiprazole hydrochloride and preparation method thereof
InactiveCN104829603ACrystal stableImprove solubilityNervous disorderOrganic chemistrySolubilityX-ray
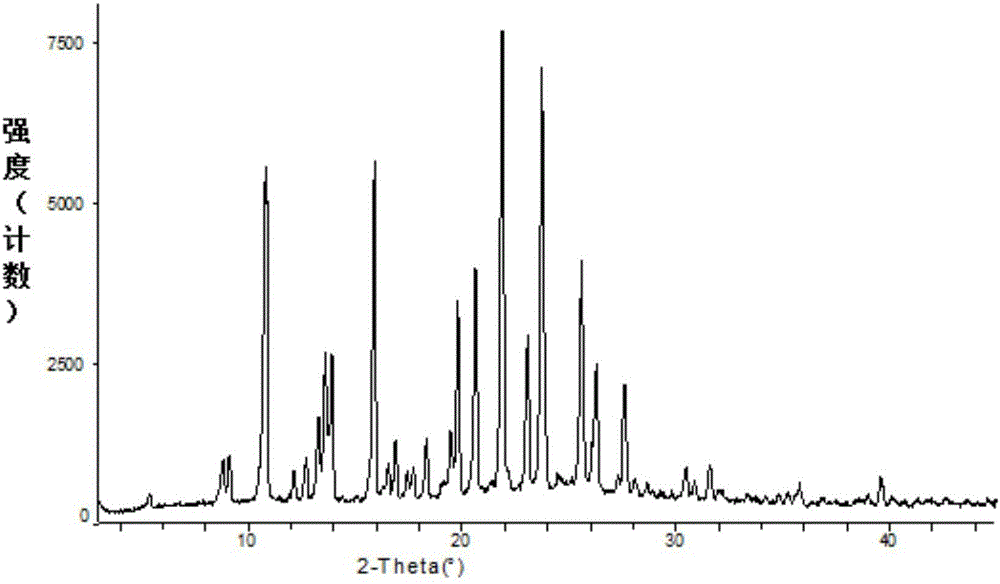
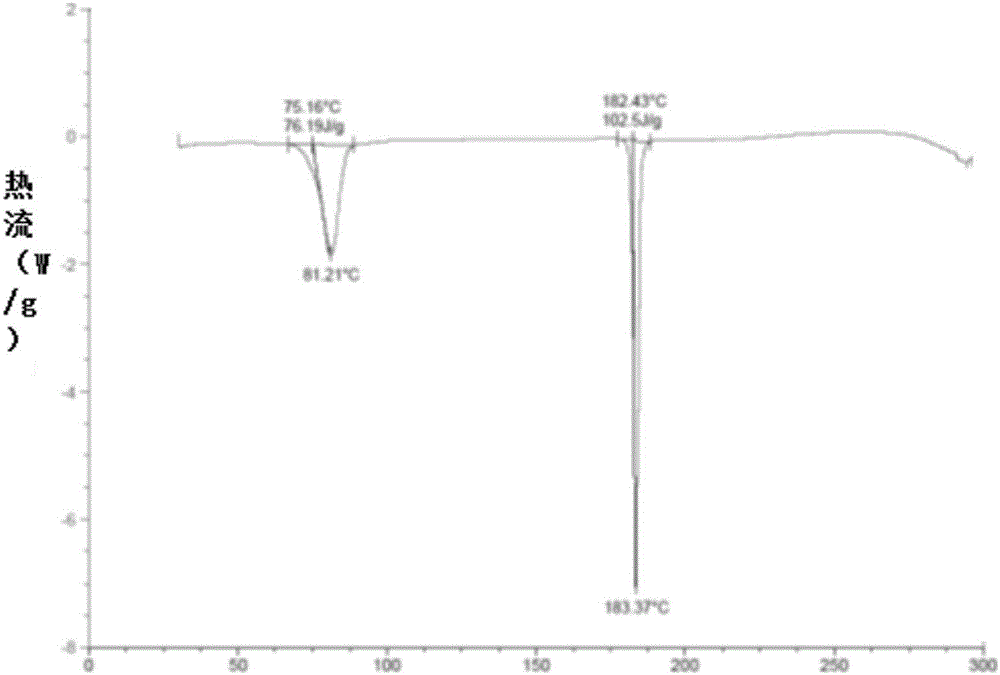
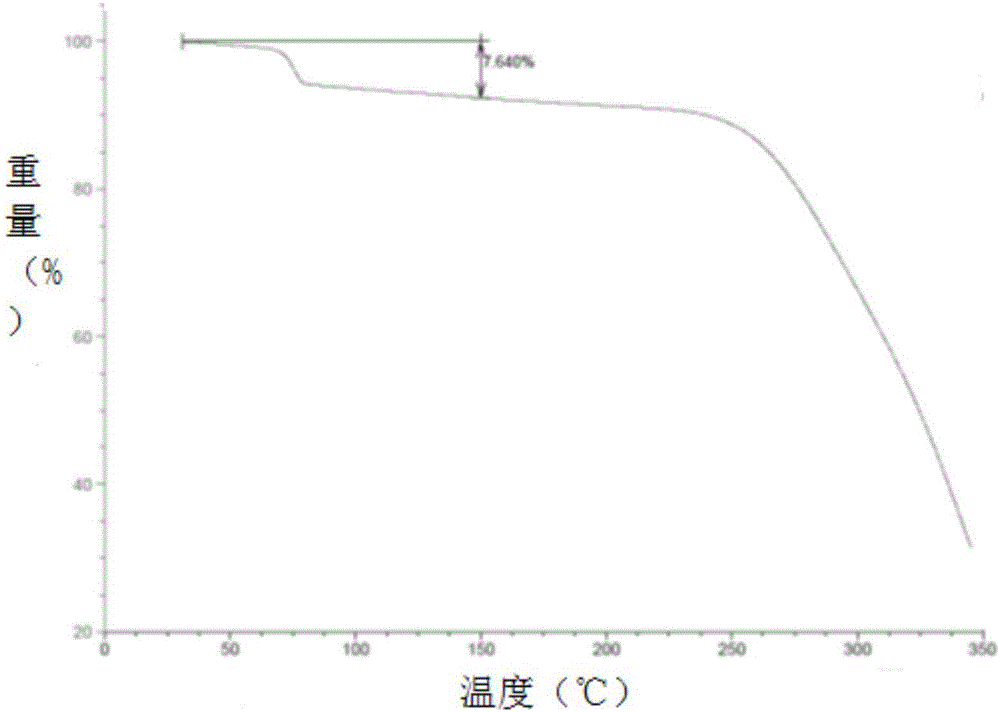
The invention relates to a crystal form A brexpiprazole hydrochloride shown in the formula (I) and a preparation method thereof. The crystal form A brexpiprazole hydrochloride is a monohydrate of brexpiprazole hydrochlorides and the powder x-ray diffraction pattern of the crystal form A brexpiprazole hydrochloride has characteristic peaks at least at the angle 2 theta of 9.2 degrees+ / -0.2 degree, 16.0 degrees+ / -0.2 degree, 17.6 degrees+ / -0.2 degree, 20.2 degrees+ / -0.2 degree, 23.8 degrees+ / -0.2 degree and 25.1 degrees+ / -0.2 degree. The preparation method comprises the following steps: (1) adding solid brexpiprazole into a mixes solvent of alcohol and organic carboxylic acid and heating for dissolving the solid brexpiprazole; (2) adjusting concentrated hydrochloric acid to adjust pH to 2-4 and cooling for crystallizing; and (3) filtering and separating the solid obtained in the step (2) and drying to obtain the crystal form A brexpiprazole hydrochloride. The crystal form A brexpiprazole hydrochloride has good solubleness and is beneficial to medicine absorption and the preparation process is simple and safe. (The formula (I) is shown in the specification).
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
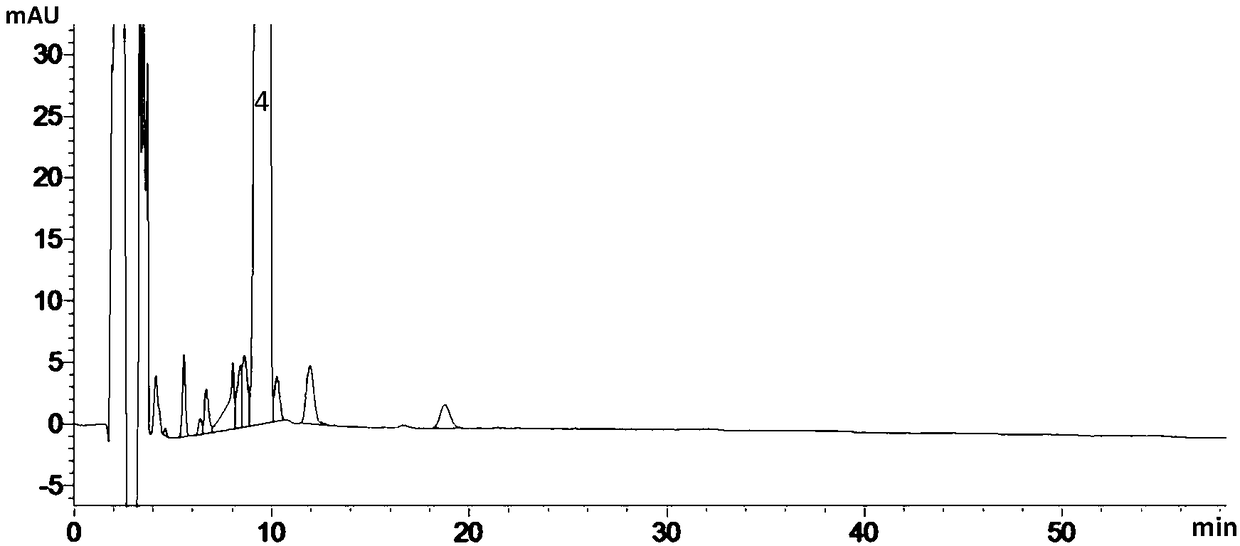
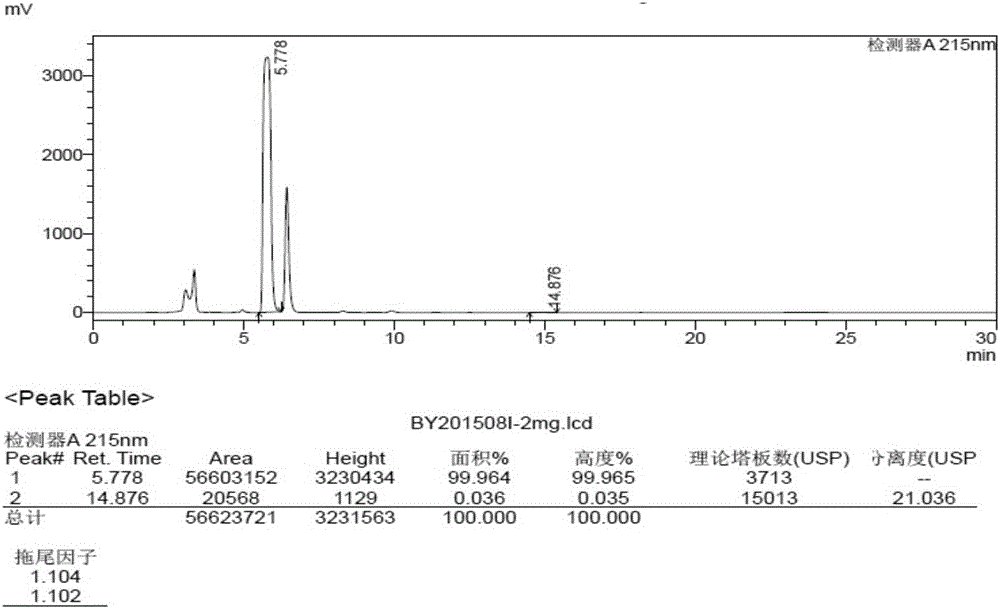
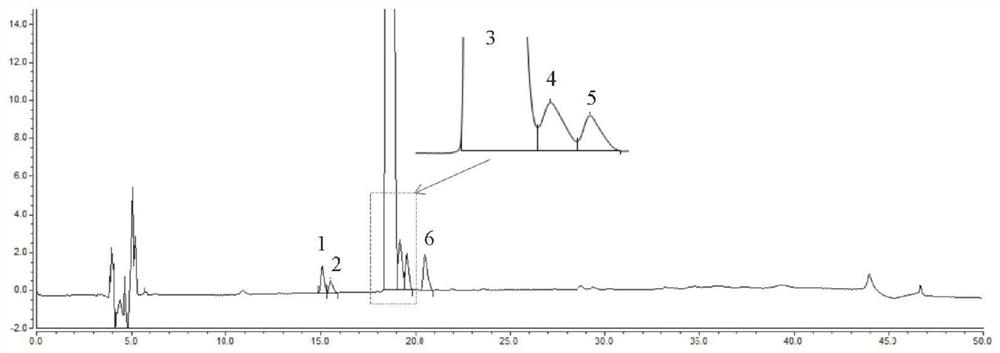
Detection method of brexpiprazole-related substance(s)
ActiveCN109307716AThe detection method is simple and fastStrong specificityComponent separationPhosphateSilanes
The present invention provides a detection method of brexpiprazole-related substance(s). The detection of the related substances is performed via a high performance liquid chromatography method. The determination condition of the liquid chromatography is: an octadecyl silane bonded silica gel is taken as a filler of a chromatographic column, a mobile phase A is acetonitrile, a mobile phase B is phosphate buffer-acetonitrile, and the detection is performed by adopting gradient elution. The method has the advantages of specificity, durability, good reproducibility and high sensitivity, and the method is suitable for the qualitative and quantitative detection of the brexpiprazole-related substance(s).
Owner:四川弘远药业有限公司
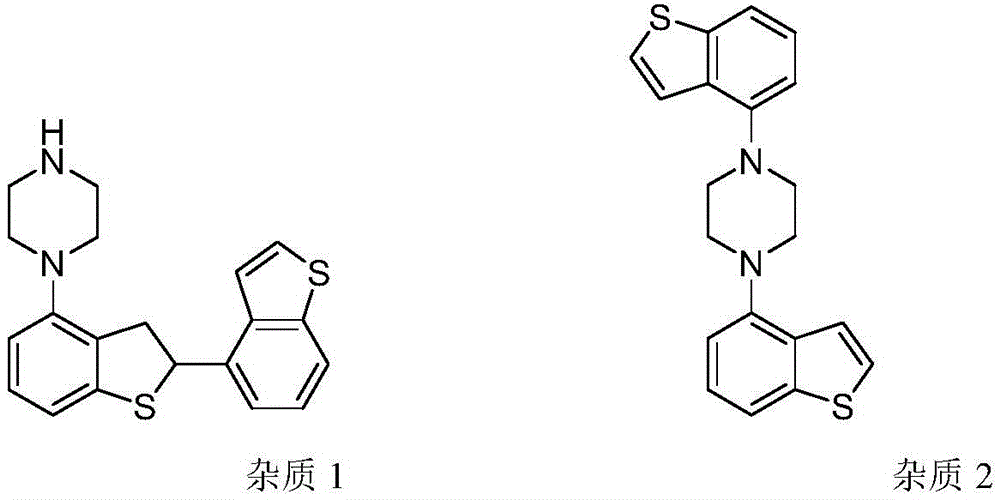
Brexpiprazole impurity compound and preparation method thereof
The invention discloses a brexpiprazole impurity compound and a preparation method thereof. The impurity compound can be one or more of a compound I, a compound II, a compound III and a compound IV. The invention also discloses a use of the impurity compound in the control of the quality of brexpiprazole.
Owner:CHONGQING PHARMA RES INST
Method for separation and determination of brexpiprazole and impurities thereof by liquid chromatography
ActiveCN107525877AEfficient separationAccurate measurementComponent separationAcetonitrileGradient elution
The invention discloses a method for separation and determination of brexpiprazole or preparations and impurities thereof by liquid chromatography. According to the method, a chromatographic column takes octadecylsilane bonded silica gel as a filler, a buffer solution is used as a mobile phase A, a mixed solvent of methanol and acetonitrile is used as a mobile phase B, the mobile phases adopt a gradient elution method, and the brexpiprazole and preparation impurities are determined. The method can effectively separate and determine the brexpiprazole with known impurities and unknown impurities. The method has the advantages of high specificity, high accuracy and easy operation, and can effectively control the quality of the pipiprazole and the preparations thereof.
Owner:CHONGQING PHARMA RES INST
Preparation method of novel brexpiprazole, aripiprazole and salts thereof
The invention provides a preparation method of novel Brexpiprazole and salts thereof, aripiprazole and salts thereof, or key intermediates thereof. The method uses a starting material of 7-hydroxycoumarin or 7-hydroxy dehydrocoumarin, which is subjected to a substitution reaction, a dehydrogenation reaction and / or amination reaction. The method has the advantages of sufficient supply of easily available raw materials, low cost, high safety, simple operation, high product yield and good quality, and is suitable for enlarge production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD +1
Method for detecting 1-bromo-4-chlorobutan in brexpiprazole intermediate
ActiveCN106770746AThe test result is accurateReliable test resultsComponent separationSilica gelRepeatability
The invention discloses a method for detecting 1-bromo-4-chlorobutan in a brexpiprazole intermediate. According to the method, by adopting a chromatographic column of octadecyl silane bonded silica gel, and the 1-bromo-4-chlorobutan in the brexpiprazole intermediate can be effectively separated and detected. A detection result is accurate and reliable, and the method has multiple advantages such as excellent linear relation, high precision, good stability, good repeatability, high recovery and simple and convenient operation, and is suitable for being popularized and applied.
Owner:CHENGDU BAIYU PHARMA CO LTD
Prophylactic and/or therapeutic agent for behavioral and psychological symptoms associated with neurodegenerative disease or impulsive symptoms associated with mental disease containing brexpiprazole or salt thereof
InactiveUS20170258787A1Good treatment effectGood effectOrganic active ingredientsNervous disorderDiseaseClinical psychology
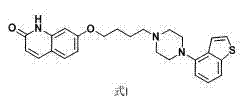
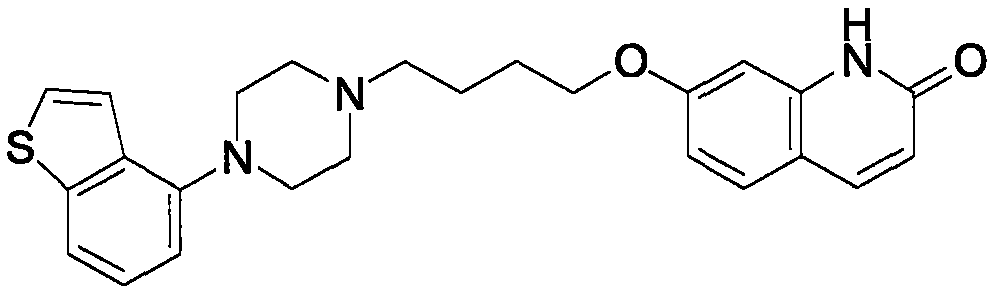
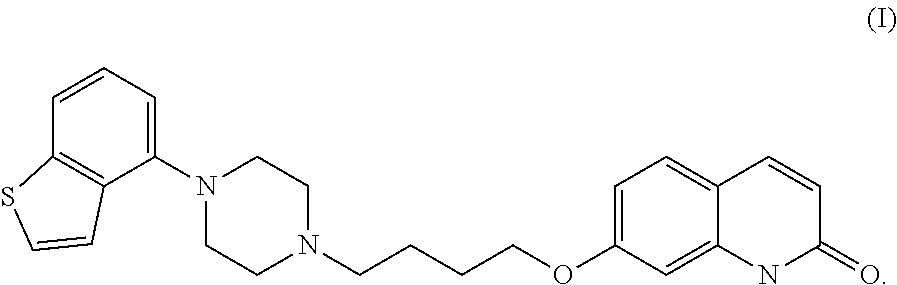
The present invention relates to a prophylactic and / or therapeutic agent for behavioral and psychological symptoms associated with neurodegenerative disease or impulsive symptoms associated with mental disease, which contains 7-[4-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)butoxy]-1H-quinolin-2-one or a salt thereof as an active ingredient.
Owner:OTSUKA PHARM CO LTD
Detection method of brexpiprazole starting material related substances
ActiveCN106645494ADetermination of comprehensiveStrong specificityComponent separationBrexpiprazoleRepeatability
The invention discloses a detection method of brexpiprazole starting material related substances. The detection method comprises steps as follows: a, preparation of a reference substance solution; b, measurement of the reference substance solution; c, preparation of a test solution; d, measurement of the test solution. With the adoption of the detection method of brexpiprazole starting material related substances, through screening with multiple processes, impurities A1-1, A1-2, A1-3 and A1-4 in a brexpiprazole starting material 7-hydroxy-2-quinolone can be effectively separated and detected, the specificity is high, detection results are accurate and reliable, and the method has multiple advantages of being high in precision, good in stability, good in repeatability, convenient to operate, shorter in time and the like. Compared with existing methods, the method has the advantages that various impurities of samples can be measured more comprehensively, the detection time is appropriate, the results are more reliable, and the method is suitable for popularization and application.
Owner:CHENGDU BAIYU PHARMA CO LTD
Preparation method and intermediate of brexpiprazole and preparation method of intermediate
ActiveCN105541819AEasy to separateReduce generationOrganic chemistryOrganic compound preparationChemical synthesisHalogen
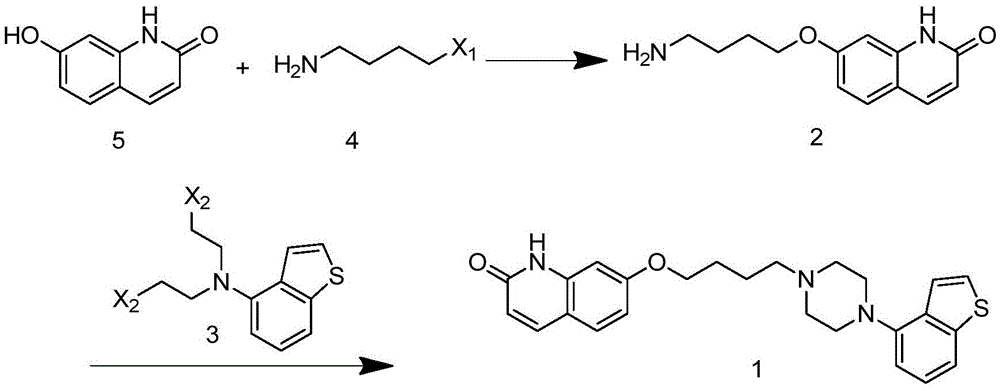
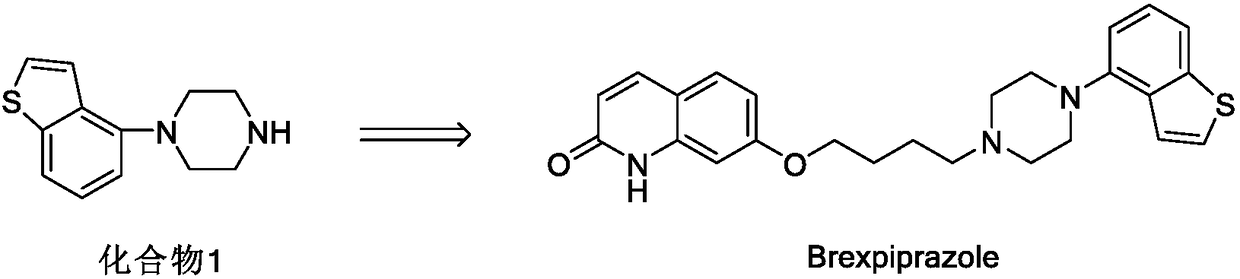
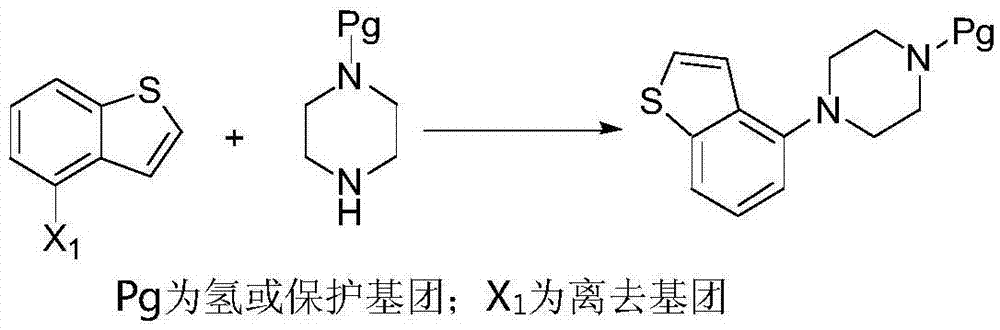
The invention relates to the technical field of medicinal chemical synthesis and in particular relates to a preparation method and an intermediate of brexpiprazole and a preparation method of the intermediate. The preparation method of the brexpiprazole is shown in the following flow chart (described in the specification), wherein X1 and X2 are halogen, X1 is bromine preferably, and X2 is chlorine preferably. The invention provides a preparation method of a new compound 1 by providing new compounds 2 and 3. The preparation method of the new compound 1 mainly comprises two-step reaction, namely substitution and cyclization. Byproducts produced in the preparation method of the new compound 1 are less and are easy to separate, and column chromatography treatment does not need to be carried out. Meanwhile, the invention also provides a preparation method of the compound 2. The preparation method of the compound 2 has the advantages that generation of byproducts is greatly reduced, overall yield of reaction is improved, column chromatography treatment does not need to be carried out and production time is shortened while production cost is reduced, so that the preparation method of the compound 2 is applicable to industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Preparation method of benzothiophene compound intermediate
InactiveCN109422722AHigh cost of industrializationImprove conversion rateOrganic chemistryState of artBenzothiophene
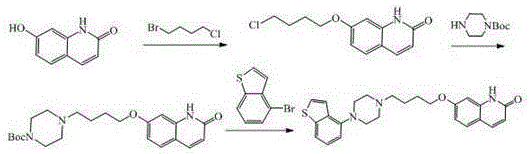
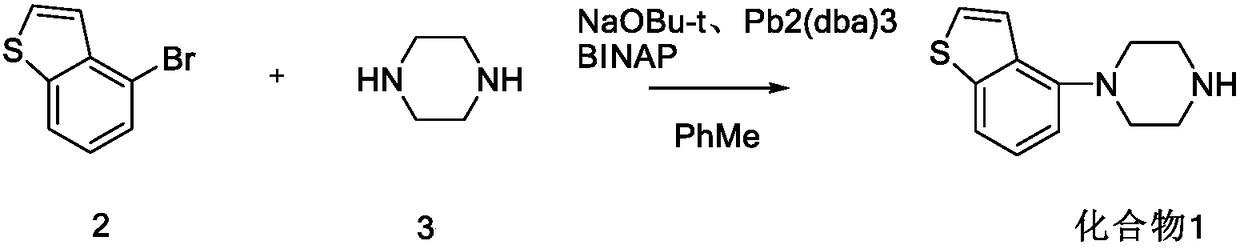
The invention provides a preparation method of a novel brexpiprazole intermediate. The method is as below: in the presence of a cuprous catalyst and a ligand, carrying out a coupling reaction on witha compound 4-halobenzo[b]thiophene and N-protecting piperazine through alkali. Compared with the prior art, the method replaces precious metal palladium with cheap copper iodide under the suitable ligand, and obtains the brexpiprazole intermediate compound with high conversion rate; and the method has the advantages of safer and more convenient reaction, low cost, less environmental pollution andeasy operation, and can be applied to industrialized production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Methods for preparing brexpiprazole intermediate and brexpiprazole with cheap metal copper
Methods for preparing a brexpiprazole intermediate and brexpiprazole with cheap metal copper are disclosed. The method for preparing the brexpiprazole intermediate includes dissolving 4-halogen benzo[b]thiophene and piperazine into a solvent under nitrogen protection, and adding an alkali, a ligand and a copper-based catalyst; stirring and reacting the mixture until TLC detection shows that the reaction is finished; after the reaction is finished, subjecting the system to extraction with ethyl acetate or dichloromethane; combining organic phases, and spin-drying the mixture; adding methanol todissolve solid; adding dropwise a hydrochloric acid methanol solution with a concentration of 2M to adjust the pH to 1-7 so that a target product forms a salt and then is precipitated; performing suction filtration to obtain white solid; and drying the white solid to obtain 4-piperazinyl benzothiophene hydrochloride. The methods have advantages of few side reactions, high product quality, low production cost, and the like, are suitable for industrial production and have good promotion and application prospects. The invention further provides a brexpiprazole bulk pharmaceutical chemical prepared from the brexpiprazole intermediate obtained with a low-cost technique, with the bulk pharmaceutical chemical having high purity.
Owner:ZHEJIANG UNIV OF TECH
Brexpiprazole-containing freeze-dried oral preparation and preparation method thereof
ActiveCN105106142AReduce weightReduce moisture contentPowder deliveryOrganic active ingredientsPatient complianceFreeze-drying
The invention provides a brexpiprazole-containing freeze-dried oral preparation and a preparation method thereof and belongs to the field of medicinal preparations. The freeze-dried oral preparation comprises brexpiprazole and pharmaceutic adjuvants in medical dosage. The preparation method for the freeze-dried oral preparation comprises the step that raw materials and auxiliary materials are used to prepare the freeze-dried oral preparation through a freeze-drying method. According to the invention, since brexpiprazole crude drug is used to prepare the freeze-dried oral preparation, the blank of brexpiprazole in the technical filed of freeze-dried preparations is filled, diverse selection of dosage forms is provided for clinical patients, and in addition, the brexpiprazole-containing freeze-dried oral preparation can be rapidly disintegrated, therefore, brexpiprazole is dissolved out quickly and quickly absorbed by patients, and bioavailability is improved; meanwhile, the freeze-dried oral preparation can be disintegrated in the mouth of the patient without water, so that partial medicines can be transferred through the mucous membrane and then absorbed, therefore, the partial medicines are absorbed before reaching the stomach, the medicine is prevented from stimulating and damaging the intestines and stomach and clinical patient compliance is improved.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Method for detecting brexpiprazole related substance
ActiveCN111912914AAccurate measurementDetermination of comprehensiveComponent separationSilanesGradient elution
The invention provides a method for detecting a brexpiprazole related substance, and the method adopts a chromatographic column with octadecylsilane chemically bonded silica as a filler, an acetonitrile solution as a mobile phase A and an ammonium chloride solution as a mobile phase B, and adopts gradient elution to determine brexpiprazole and related substances thereof. According to the method, brexpiprazole can be effectively separated from adjacent impurities and various impurities, the separation degree is high, brexpiprazole and related substances thereof can be accurately detected, the problem that brexpiprazole and related substances thereof are difficult to separate and detect is effectively solved, and therefore it is guaranteed that the quality of brexpiprazole is controllable.
Owner:四川弘远药业有限公司
Pharmaceutical composition containing brexpiprazole and amphiphilic polymer as well as preparation method and application of pharmaceutical composition
PendingCN113082004ASolution to slow dissolutionImprove bioavailabilityOrganic active ingredientsNervous disorderPolymer sciencePharmaceutical Substances
The invention provides a pharmaceutical composition containing brexpiprazole and an amphiphilic polymer as well as a preparation method and application of the pharmaceutical composition. The pharmaceutical composition comprises brexpiprazole and an amphiphilic polymer, and the brexpiprazole is wrapped in a lipophilic core of an amphiphilic polymer micelle to form a brexpiprazole-amphiphilic polymer inclusion compound. Through the above mode, the solubility of the insoluble drug brexpiprazole in an aqueous medium can be greatly improved, so that the prepared pharmaceutical composition has shorter disintegration time limit and faster dissolution rate, and the bioavailability of the drug is effectively improved. Meanwhile, the pharmaceutical composition containing the brexpiprazole and the amphiphilic polymer is prepared into an oral cavity instant film agent, so that the oral cavity instant film agent can be quickly dissolved on a tongue and can be taken without water, so that the medication compliance and convenience of patients are effectively improved. The preparation method provided by the invention is convenient to operate, good in reproducibility, strong in controllability and relatively low in cost, and has good social benefits and economic benefits.
Owner:江苏谛奇医药科技有限公司
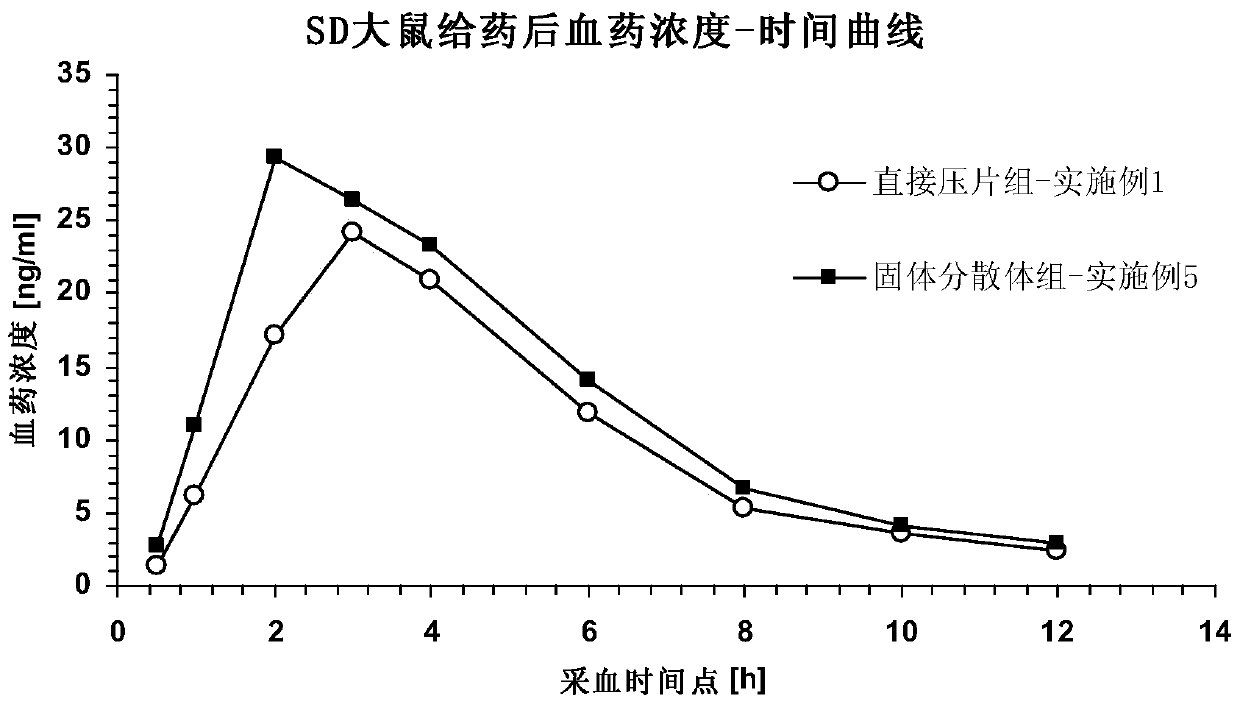
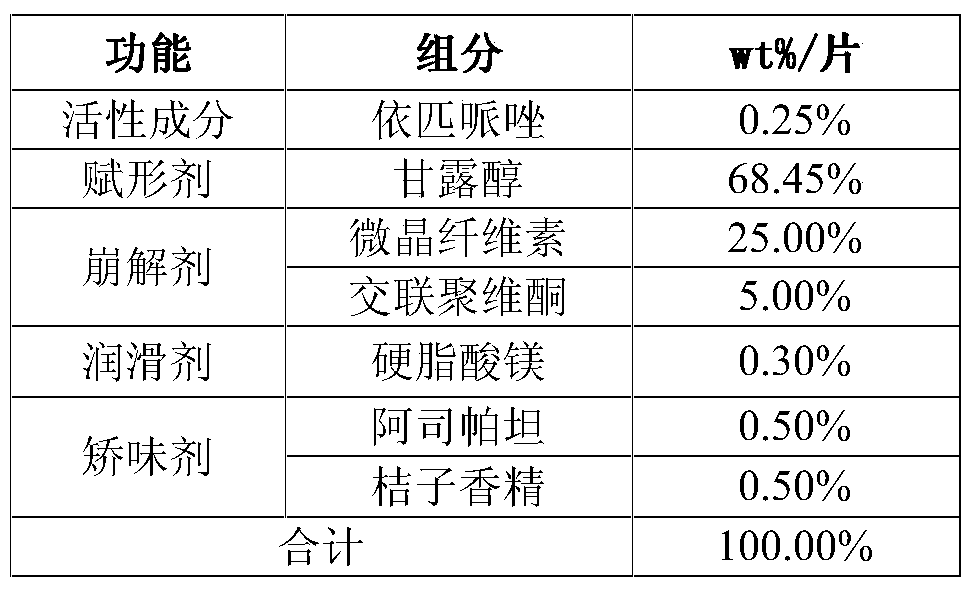
Preparation method of brexpiprazole tablets
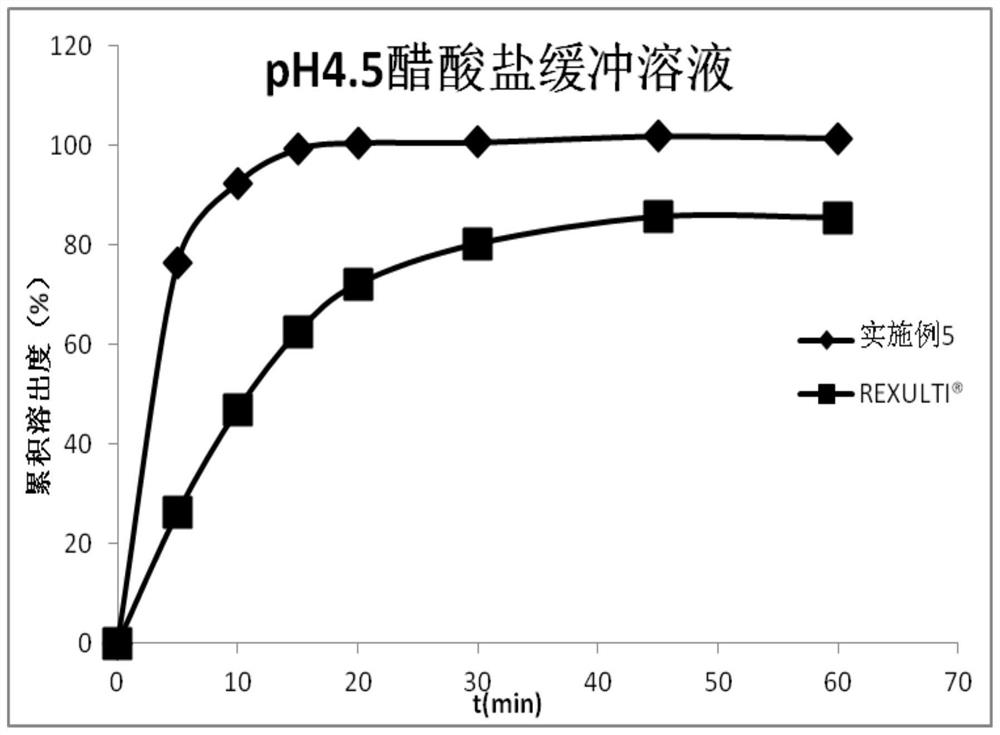
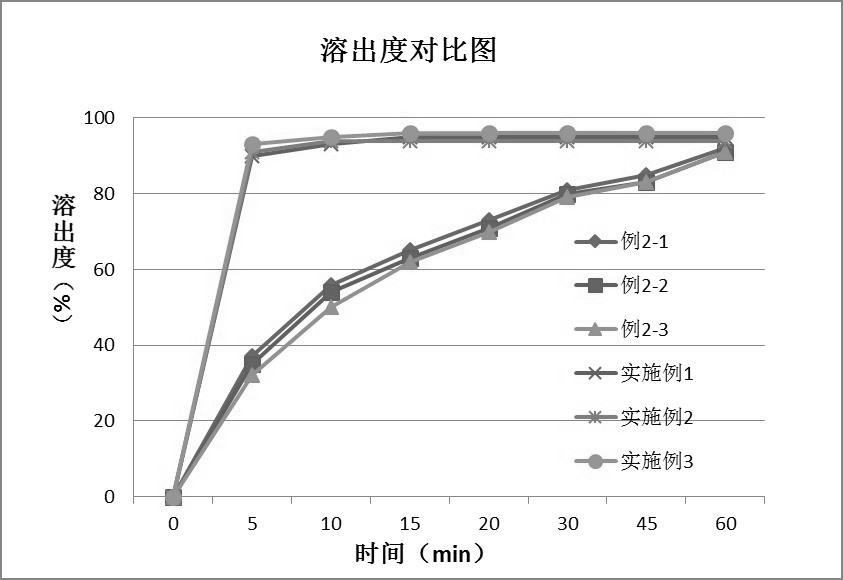
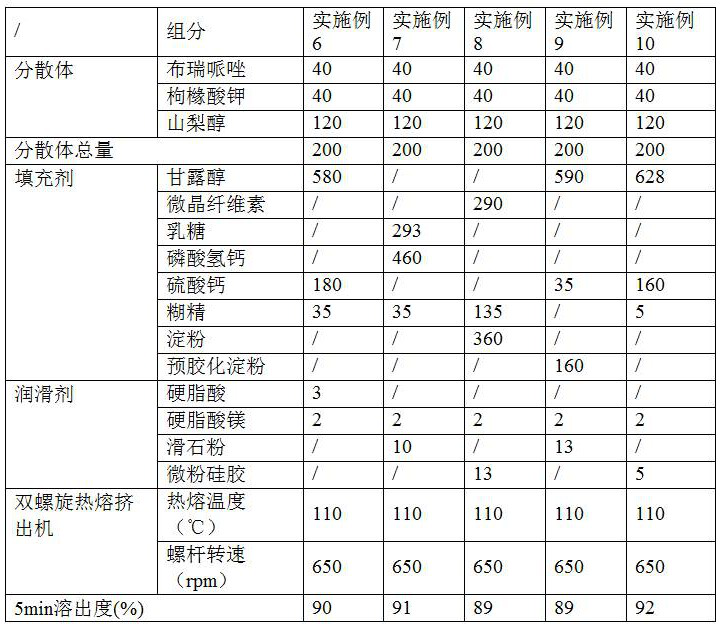
PendingCN112168794AInhibition of high temperature degradationLower requirementOrganic active ingredientsNervous disorderHot meltPharmaceutical Aids
The present invention discloses a preparation method of brexpiprazole tablets and belongs to the field of pharmaceutical preparations. The brexpiprazole tablets are prepared by directly tabletting brexpiprazole solid dispersion particles and auxiliary materials, and the brexpiprazole solid dispersion particles are prepared by the following method: heating and melting potassium citrate, sorbitol and brexpiprazole in a hot melt extruder, and extruding and granulating the melt materials, wherein a weight ratio of brexpiprazole to potassium citrate is 1:(0.5-2) and a weight ratio of the brexpiprazole to the sorbitol is 1:(1-5). An in-vitro dissolution rate of the brexpiprazole is increased.
Owner:浙江诺得药业有限公司 +1
Preparation method of brexpiprazole, and compound used for preparing brexpiprazole
InactiveCN106938982AEasy to produceAtom utilization is highOrganic chemistryBulk chemical productionBrexpiprazoleMedicinal chemistry
The invention discloses a preparation method of brexpiprazole, and a compound used for preparing brexpiprazole. A compound IV reacts with a compound VIII to obtain the brexpiprazole. The preparation method has the advantages of cheap and easily available raw materials, simple synthesis technology, greenness and environmental protection, and suitableness for industrial production.
Owner:连云港皓海医药科技有限公司
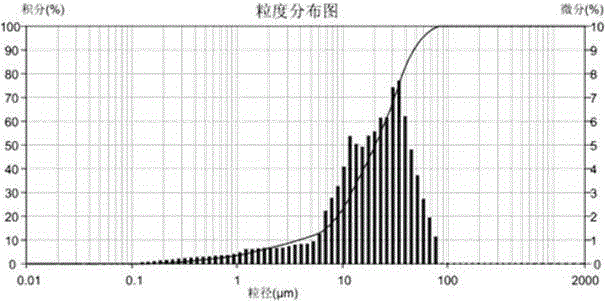
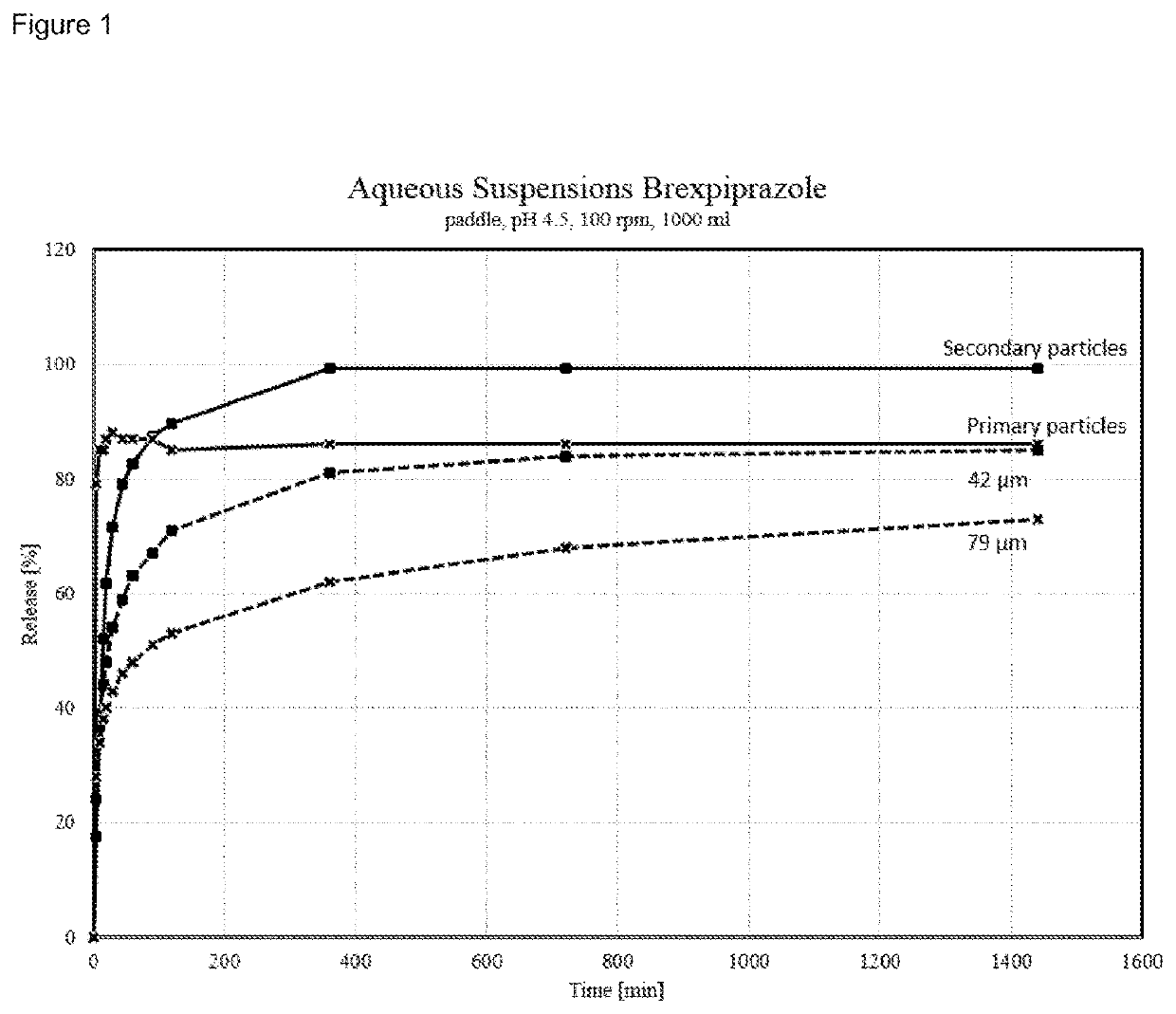
Crystalline Brexpiprazole
ActiveUS20190389847A1Increased complexityImproved profileNervous disorderOrganic chemistry methodsCrystallographyPrefilled Syringe
The present invention relates to crystalline brexpiprazole having a particle size distribution (PSD) characterized by a d(90) of 30 μm to 100 μm, by a d(10) of at least 1.0 μm, and by a d(4,3) of at least 15.0 μm. The present invention also relates to a pharmaceutical composition comprising a crystalline brexpiprazole having a PSD characterized by a d(90) of 30 μm to 100 μm, by a d(10) of at least 1.0 μm, and by a d(4,3) of at least 15.0 μm, and to an aqueous suspension comprising said crystalline brexpiprazole. The present invention also relates to a composition comprising a crystalline brexpiprazole having a PSD characterized by a d(90) of 30 μm to 100 μm, by a d(10) of at least 1.0 μm, and by a d(4,3) of at least 15.0 μm, wherein said composition is essentially free from secondary particles of brexpiprazole. The present invention also relates to an injectable preparation, vial, prefilled syringe, or kit, comprising crystalline brexpiprazole having a PSD characterized by a d(90) of 30 μm to 100 μm, by a d(10) of at least 1.0 μm, and by a d(4,3) of at least 15.0 μm. The present invention also relates to a process for the preparation of an aqueous suspension, a vial, a prefilled syringe, or a lyophilisate, comprising a step of incorporating crystalline brexpiprazole having a PSD characterized by a d(90) of 30 μm to 100 μm, by a d(10) of at least 1.0 μm, and by a d(4,3) of at least 15.0 μm. Finally, the present invention also relates to crystalline brexpiprazole having a PSD characterized by a d(90) of 30 μm to 100 μm, by a d(10) of at least 1.0 μm, and by a d(4,3) of at least 15.0 μm, for use in the treatment or prevention of relapse of schizophrenia, bipolar disorder, or depression.
Owner:HEXAL AG
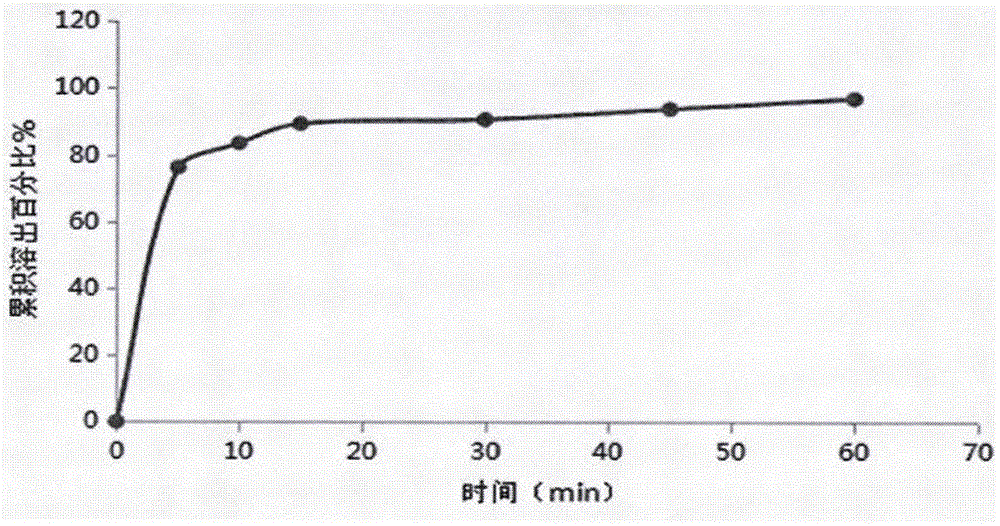
Technology for improving solubility of brexpiprazole and method for preparing brexpiprazole tablet
The invention relates to a technology for improving the solubility of insoluble medicine of brexpiprazole and preparation of a brexpiprazole tablet, and belongs to the technical field of pharmaceutical preparations. Brexpiprazole is used as a medicinal active component for jet milling, and then a hydrophilic auxiliary material is used for preparing a solid dispersion, so that the highly dispersedstate of the medicine can be ensured; the solubility and the dissolution rate of insoluble medicine are effectively improved; and the bioavailability of the medicine is improved. Then, the solid dispersion is further prepared into tablets; the medicine absorption in the body is promoted; the bioavailability of the medicine is further improved; in addition, the preparation process is simple; dust pollution is avoided; industrial production is easy; and meanwhile, the medicine taking compliance of a patient is enhanced.
Owner:BEIJING VENTUREPHARM BIOTECH
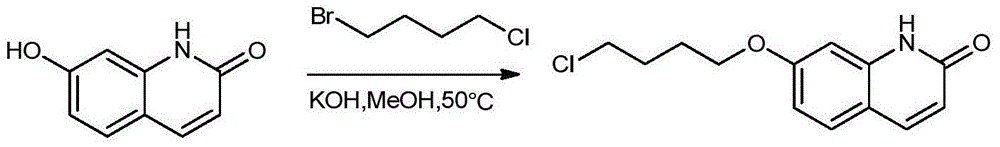
Synthesis method of brexpiprazole intermediate 7-(4-chlorobutoxy)-1H-quinoline-2-one
InactiveCN106008337AHigh purityShort reaction timeOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis and particularly relates to a synthesis method of a brexpiprazole intermediate 7-(4-chlorobutoxy)-1H-quinoline-2-one. The method comprises the following steps: 7-hydroxyl-1H-quinoline-2-one and 1-bromo-4-chlorobutane react in a solvent under the function of triethylamine, crude 7-(4-chlorobutoxy)-1H-quinoline-2-one is obtained and re-crystallized, and refined 7-(4-chlorobutoxy)-1H-quinoline-2-one is obtained. According to the method, the reaction effect is good, the product yield is high, the purity is high, the cost is low, operation is simple, and the method is especially suitable for industrial production.
Owner:山东川成医药有限公司
Brexpiprazole purifying method
The invention belongs to the technical field of chemical synthesis, and concretely relates to a brexpiprazole purifying method. The method comprises the following steps: mixing crude brexpiprazole with acetone, heating the obtained mixture until the mixture is refluxed, stirring until solid is dissolved, adding active carbon to decolorize, carrying out hot filtration, naturally cooling the obtained filtrate to room temperature, carrying out heat insulation crystallization, and washing the obtained filtered solid with cold acetone to obtain purified brexpiprazole. The brexpiprazole purifying method has the advantages of good purifying effect, high product yield, low cost, and simplicity in operation, and is especially suitable for industrial production.
Owner:山东川成医药有限公司
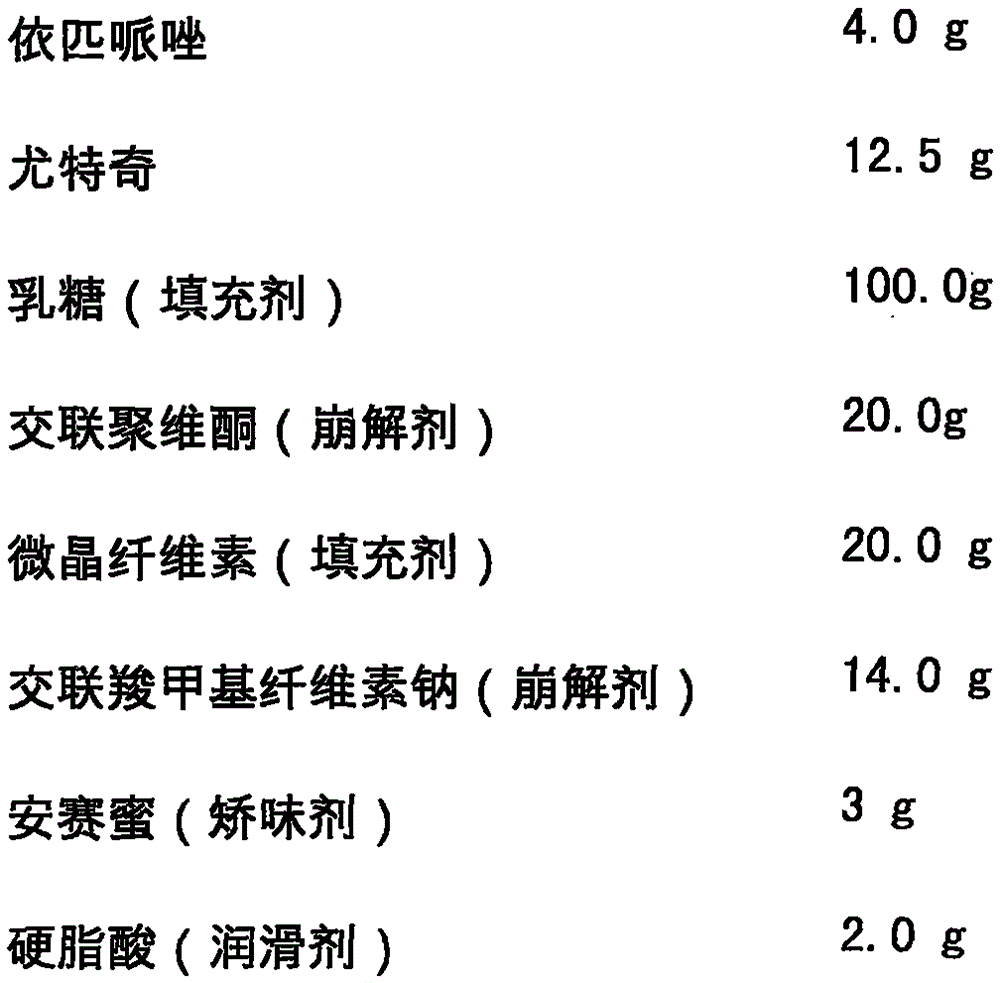
Orally disintegrating tablet containing Brexpiprazole or salt and preparation method thereof
InactiveCN106994119ADisintegrates quicklyGreat tasteOrganic active ingredientsPill deliveryActive componentOrally disintegrating tablet
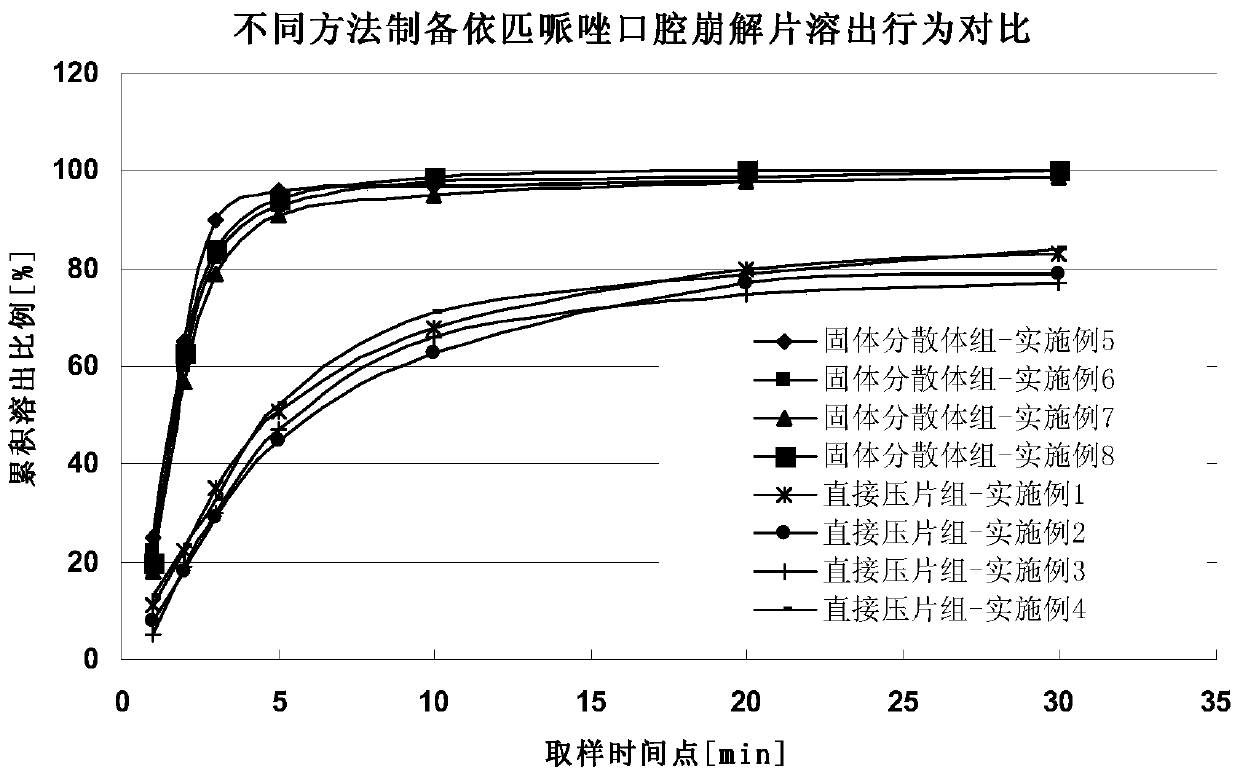
The invention relates to an orally disintegrating tablet containing Brexpiprazole or salt and also relates to a preparation method thereof. The orally disintegrating tablet contains 0.06-15.0% of Brexpiprazole, 40.0-95.0% of an excipient, 3.0-50.0% of a disintegrating agent, 0.0-6.0% of a lubricant and 0.05-5.0% of a corrective agent. The orally disintegrating tablet is prepared by the following steps: firstly preparing a solid dispersion from the active component Brexpiprazole; and then mixing the active component Brexpiprazole with other auxiliary materials. The orally disintegrating tablet has the following advantages: disintegration is fast; mouthfeel is good; bioavailability is high; quality is stable and controllable; auxiliary materials are safe and easily available; the preparation method is simple; no expensive special equipment is required; and the product is easy to store and transport.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Brexpiprazole methyl alcohol compound, crystal form A and preparation method and application
InactiveCN106699745AFacilitate dissociationImprove solubilityOrganic active ingredientsNervous disorderSolubilityAlcohol
The invention discloses a brexpiprazole methyl alcohol compound, a crystal form A and a preparation method and application. The crystal form A of the brexpiprazole methyl alcohol compound has characteristic peaks at the positions of 3375.1 cm-1, 3064.3 cm-1, 2942.3 cm-1, 2819.0 cm-1, 1648.3 cm-1, 1625.0 cm-1, 1449.7 cm-1, 1220.8 cm-1 and 841.4 cm in an infrared absorption spectrum measured by adopting a potassium bromide pellet technique. The preparation method is safe, simple and convenient, the prepared brexpiprazole methyl alcohol compound or the crystal form A is better in solubility, makes dissociation easy so as to obtain brexpiprazole, is good in slow-release effect, suitable for drug development and wide in market prospect.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com