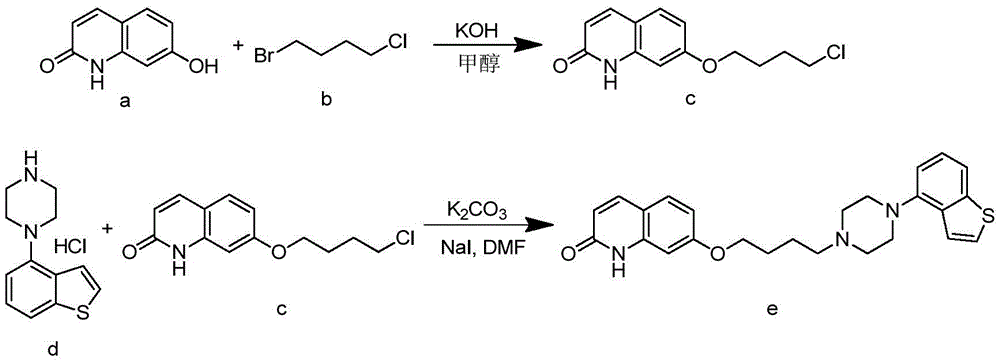
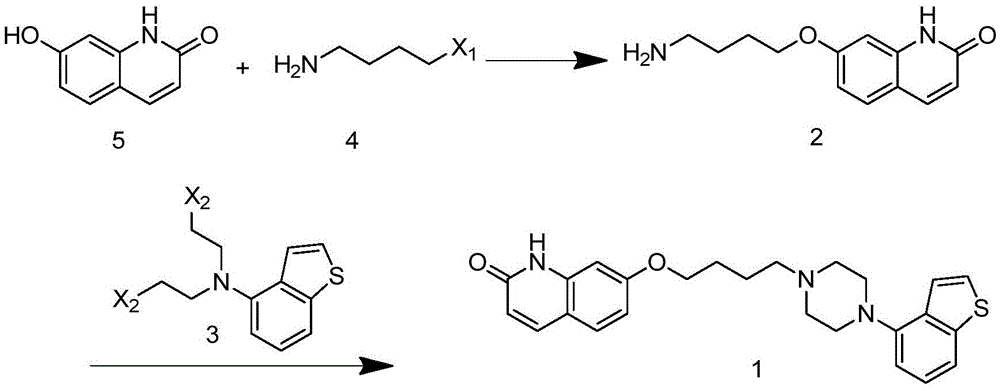
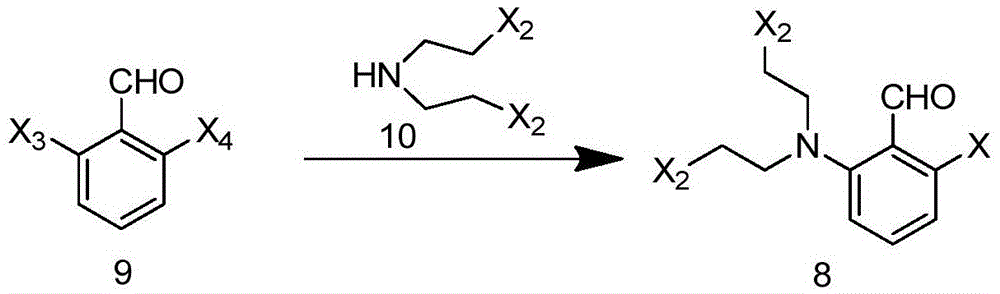
Preparation method and intermediate of brexpiprazole and preparation method of intermediate
A kind of technology of epizole and compound, applied in the field of pharmaceutical chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: the preparation of compound 8a
[0083]
[0084] At room temperature, in a 100ml three-necked flask, add compound 9a (40.6g, 0.20mol), bis(2-chloroethyl)amine (34.1g, 0.24mol), potassium carbonate (55.3g, 0.40mol), toluene (400ml), Heat up to 110°C and reflux, react for 4 hours, TLC observes that the reaction is complete; the system is cooled to room temperature, add 400ml of water, separate the layers, extract the aqueous layer with 400ml of toluene, combine the organic layers, concentrate to obtain 64.2g of solid, and use 200ml of n-heptane Make a slurry, filter, and remove the solvent from the filtrate to obtain 57.9 g of solid. The yield is 89.1%.
[0085] 1 HNMR (300MHz, CDCl 3 ): δ10.22(s,1H),7.17(t,J=14.8Hz,1H),7.07(dd,J=15.0,3.3Hz,1H),6.86(dd,J=14.7,3.4Hz,1H) ,3.85-3.53(m,8H).
Embodiment 2
[0086] Embodiment 2: the preparation of compound 7a
[0087]
[0088] At room temperature, a 500ml three-necked flask was mechanically stirred, and compound 8a (48.8g, 0.15mol), ethyl thioglycolate (21.6g, 0.18mol), N,N-dimethylformamide (675ml), potassium carbonate (45.6 g, 0.33mol), heated to 100°C, stirred and reacted for 4 hours, observed by TLC, the reaction of the raw materials was complete; cooled to room temperature, added 750ml of water, 750ml of ethyl acetate, separated, added 750ml of ethyl acetate to the water layer for extraction, and combined the organic layer, washed with 900ml of water, washed with 600ml of sodium chloride, dried over anhydrous sodium sulfate, and the solvent was removed from the organic layer to obtain 51.8g of a brown solid. Add 225ml of methyl tert-butyl ether and raise the temperature to 40°C and stir for 1 hour, then lower it to room temperature and stir for 2 Filtrate for 1 hour, remove the solvent from the filtrate, and dry to obtain ...
Embodiment 3
[0090] Embodiment 3: the preparation of compound 6a
[0091]
[0092] At room temperature, in a 250ml three-necked flask, add compound 7a (41.5g, 0.12mol), methanol 200ml, and water 40ml, cool down to 0°C, add lithium hydroxide monohydrate (10.1g, 0.24mol) in three batches, and stir for 5 minutes. Raise the temperature to 50°C and react for 3 hours. TLC observed that the reaction of the raw materials was complete. Concentrate the reaction solution under reduced pressure in a water bath at 40°C until it stopped dripping. Add 2000ml of water and adjust the pH to 2-3 with 1mol / L hydrochloric acid, and extract twice with 1200ml of ethyl acetate. , combined the organic layers, washed with 800ml of saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain off-white solid, then added 800ml of methyl tert-butyl ether for beating and filtered, the filtrate was desolventized, dried to obtain 32.5g of off-white solid, and collected The rate is 85.1%.
[0093] 1 HN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com