Method for detecting brexpiprazole related substance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
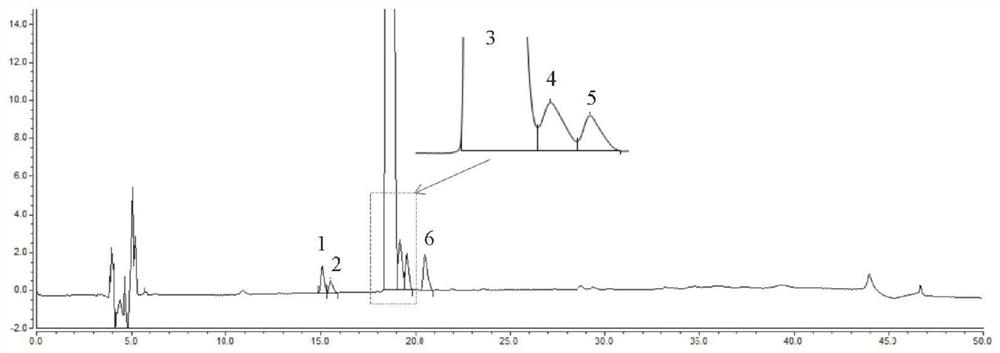
[0109] Embodiment 1 System suitability experiment
[0110] 1. Instruments and conditions
[0111] Chromatographic column: InertsilODS-3C18 column (4.6×150mm, 3μm)
[0112] Mobile phase: mobile phase A: 0.1% formic acid-acetonitrile solution, mobile phase B: 0.1% formic acid-0.04mol / L ammonium chloride solution
[0113] Flow rate: 1.0ml / min
[0114] Column temperature: 35°C
[0115] Detection wavelength: 254nm
[0116] Carry out gradient elution according to the table below:
[0117]
[0118] 2. Operation steps
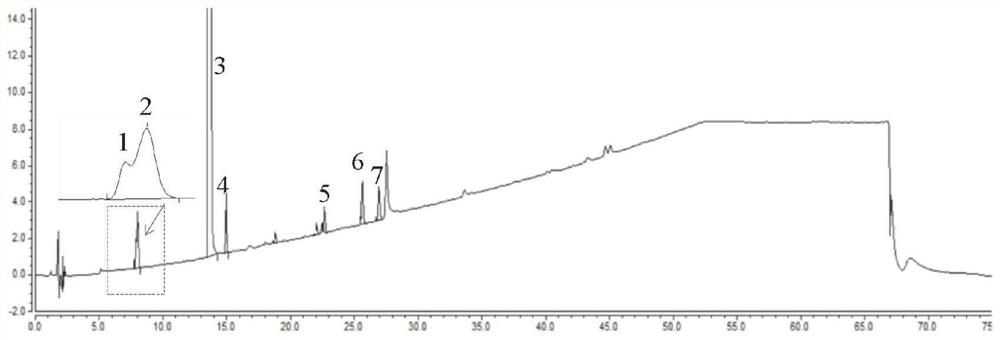
[0119] Accurately weigh appropriate amounts of ebiprazole reference substance and impurity BPZ-8, BPZ-9, BPZ-16, BPZ-23, BPZ-20, BPZ-29 reference substance, and diluent [0.02mol / LNa 2 SO 4 -acetonitrile-methanol-glacial acetic acid (56:33:11:1)] was dissolved and quantitatively diluted to make a mixed solution containing about 0.4 mg of ebiprazole and 0.8 μg of BPZ impurity per 1 ml as a system suitability solution. Inject 20 μl into the liquid chromatogra...
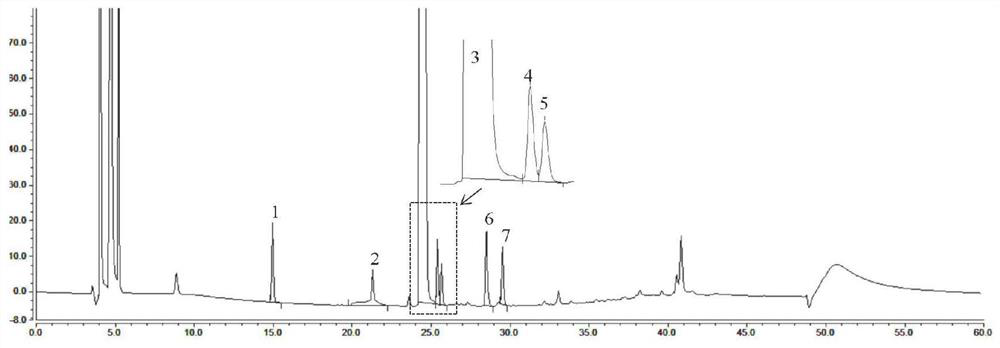
Embodiment 2
[0124] Example 2 Ebiprazole bulk drug accelerated test (40°C ± 2°C temperature, 75% ± 5% relative humidity) sample detection
[0125] 1. Instruments and conditions
[0126] Chromatographic column: InertsilODS-3C18 column (4.6×150mm, 3μm)
[0127] Mobile phase: mobile phase A: acetonitrile solution, mobile phase B: 0.04mol / L ammonium chloride solution
[0128] Wavelength: 254nm;
[0129] Flow rate: 1.0ml / min;
[0130] Column temperature: 35°C;
[0131] Injection volume: 20ul;
[0132] The elution gradient is shown in the table below:
[0133]
[0134] 2. Operation method: Accurately weigh about 10 mg of ebiprazole crude drug after the accelerated test, put it in a 25ml measuring bottle, add an appropriate amount of diluent and shake to dissolve, dilute to the mark with diluent to obtain the test solution. Precision measures need testing solution 0.5ml, puts in 100ml measuring bottle, with diluent [0.02mol / LNa 2 SO 4 -acetonitrile-methanol-glacial acetic acid (56:33:11:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com