Crystal form A brexpiprazole hydrochloride and preparation method thereof
A technology of epirazole and hydrochloride, applied in the field of medicine, can solve the problems of cumbersome preparation process of epirazole dihydrate, low solubility of epirazole anhydrous, etc., and achieves strong operability and solubility. Good properties and stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
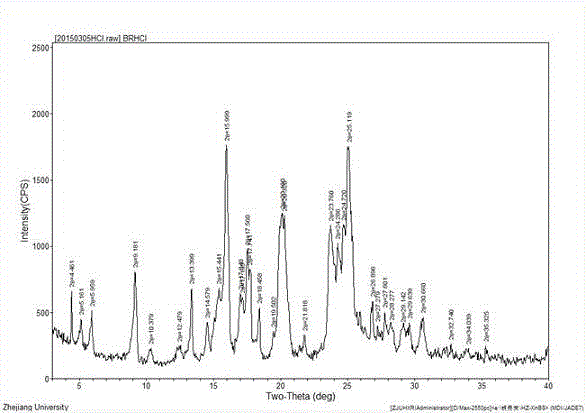
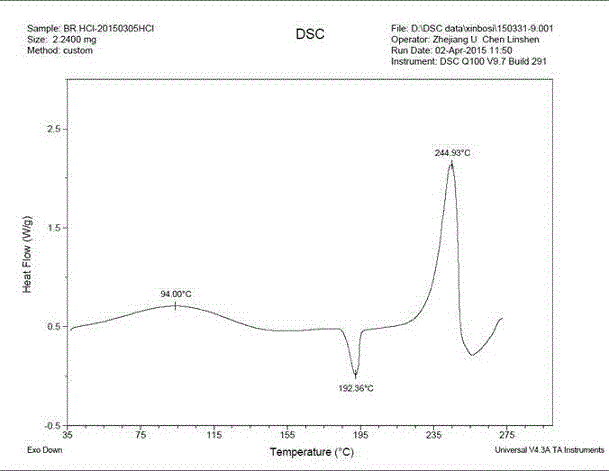
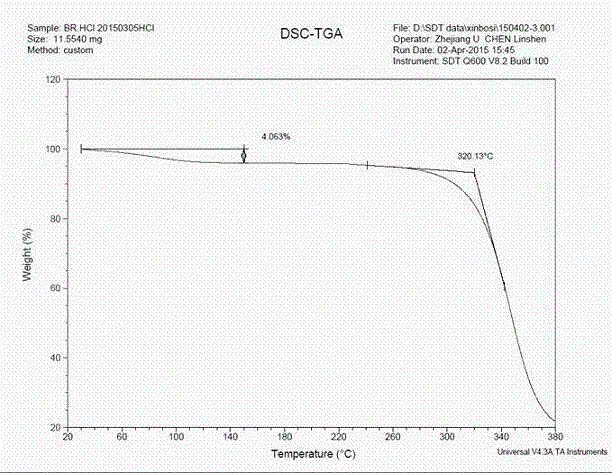
[0028] In a 250ml three-necked flask, add 7.1g of the epizole solid, add the mixture of 160ml of ethanol and 10ml of acetic acid, and heat to reflux and stir. After all the solids were dissolved, concentrated hydrochloric acid was added dropwise until the pH was 2-3. After the dropwise addition, the temperature was naturally lowered to the temperature in the system ≤ 20°C. Filter to obtain a white filter cake. Blast-dried at 70°C to obtain 7.6 g of crystalline form A epirazole hydrochloride with a molar yield of 95%. Determination of its XRPD, DSC and DSC-TGA, its spectra are as follows Figure 1-Figure 3 shown.
Embodiment 2
[0030] In the 3L four-neck flask, add 90g of the epizole solid, add the mixture of 2050ml of ethanol and 150ml of acetic acid, and heat to reflux and stir. After all the solids were dissolved, concentrated hydrochloric acid was added dropwise until the pH was 2-3. After the dropwise addition, the temperature was naturally lowered to the temperature in the system ≤ 20°C. Filter to obtain a white filter cake. Blow drying at 70°C to obtain 96 g of crystalline form A epirazole hydrochloride with a molar yield of 95%.
[0031] The chemical formula of the crystal form A epizopine hydrochloride obtained in Examples 1 and 2 is: , and compared it with the solubility of epirazole anhydrate, the results showed that: at 37°C, the saturated solubility of epirazole anhydrate in an aqueous solution with a pH value of 4.5 was 0.1 mg / ml; crystal form A Epirazole hydrochloride has a saturated solubility of 0.6 mg / ml in an aqueous solution with a pH value of 4.5. The type A crystalline e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com