Brexpiprazole preparation method
A technology of epipiprazole and compound, applied in the field of preparation of epipiprazole, can solve the problems of difficult separation of impurities, side reactions, poor reaction selectivity, etc., and achieves the effects of high reaction selectivity, easy operation and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
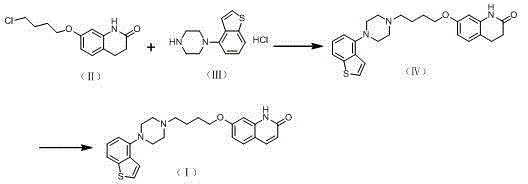
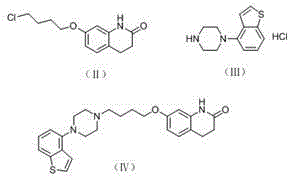
[0024] The preparation of embodiment 1 formula Ⅳ compound
[0025] Add 4ml of N-methylpyrrolidone, 0.5g of the compound of formula II and 0.53g of the compound of formula III, 0.28g of sodium carbonate, and 0.2g of sodium bromide into a 25ml three-necked flask, heat up to 70-80 degrees and react for 3-5 hours. After completion, cool down to room temperature, add 12ml of water, precipitate a white solid, filter with suction, wash the filter cake twice with water, and dry under reduced pressure to obtain the compound of formula IV, with a dry weight of 0.84g. Yield: 97.8%, purity 98.3%.
Embodiment 2
[0026] The preparation of embodiment 2 formula Ⅳ compound
[0027] Add 400L of N-methylpyrrolidone, 50g of the compound of formula II and 50g of the compound of formula III, 27.6g of sodium carbonate, and 28.6g of potassium iodide into a 2L three-necked flask, heat up to 70-80°C and react for 3-5 hours. After the reaction is completed, cool down After reaching room temperature, 1.2 L of water was added to precipitate a white solid, which was filtered with suction, washed twice with water, and dried under reduced pressure to obtain the compound of formula IV, with a dry weight of 82.3 g. Yield: 95.8%, purity 97.6%.
Embodiment 3
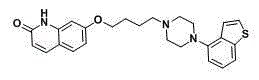
[0028] The preparation of embodiment 3 ebiprazole
[0029] Add 700ml of N-methylpyrrolidone, 100g of the compound of formula IV and 67.7g of DDQ into a 1000ml three-necked flask, and stir and react with 30-40°C for 3-4 hours. In 2.5L aqueous solution, after stirring for 0.5 hour, suction filtration, the filter cake was washed three times with water, and dried under reduced pressure to obtain ebiprazole, off-white powdery solid, 93.6g, yield 94.0%, purity 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com