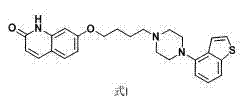
Preparation method of brexpiprazole
A technology of ebiprazole and compounds, applied in the field of medicinal chemistry, can solve the problems of low purity of intermediates, many by-products, and long reaction time, and achieve the effects of high reaction selectivity, reduced raw material costs, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
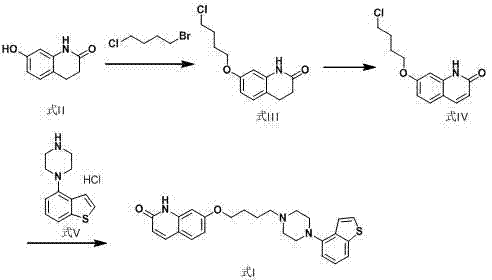
[0026] Example 1 7-(4-chlorobutoxy)-3,4-dihydroquinoline-2(1 H )-Preparation of ketone (formula III)
[0027] Add 10g of 3,4-dihydro-7-hydroxy-2(1H)-quinolinone (Formula II), 31.5g of 1-bromo-4-chlorobutane, 30ml of N-methylpyrrolidone, water 5ml, sodium hydroxide 2.9g. After the addition, heat up to 40-50°C, seal and keep warm for 3 hours. After the reaction, add water to crystallize, filter with suction, wash with water twice, and dry the filter cake under reduced pressure to obtain 7-(4-chlorobutoxy)-3. 4-Dihydroquinoline-2(1 H )-ketone 14.6g, yield 94.3%, purity 97.8%.
Embodiment 2
[0028] Example 2 7-(4-chlorobutoxy)-3,4-dihydroquinoline-2(1 H ) - Preparation of ketones
[0029] Add 3,4-dihydro-7-hydroxy-2(1H)-quinolinone 1.0kg, 1-bromo-4-chlorobutane 3.15kg, DMF 3.0L, water 500ml, lithium hydroxide 270 g. After the addition, heat up to 40-50°C, seal and keep warm for 3 hours. After the reaction, add water to crystallize, filter with suction, wash with water twice, and dry the filter cake under reduced pressure to obtain 7-(4-chlorobutoxy)-3. 4-Dihydroquinoline-2(1 H )-ketone dry product 1.48kg, yield 95.0%, purity greater than 98.5%.
Embodiment 3
[0030] Example 3 7-(4-chlorobutoxy)-quinoline-2(1 H )-Preparation of ketone (formula IV)
[0031] Add 700ml of tetrahydrofuran into a 1L three-necked flask, 7-(4-chlorobutoxy)-3,4-dihydroquinoline-2(1 H )-ketone 100g, DDQ 110g, stirred and reacted at 30-40°C for 3-4 hours, after the reaction was completed, pour the reaction solution into an aqueous solution containing 55g of sodium sulfite, stirred for 0.5 hours, then suction filtered, and the filter cake was used Washed three times, dried under reduced pressure to obtain 7-(4-chlorobutoxy)-quinoline-2(1 H )-ketone 97g, yield 98.0%, purity 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com