Preparation method of brexpiprazole, and compound used for preparing brexpiprazole
A compound and basic substance technology, applied in the field of drug synthesis, can solve problems such as long production cycle, cumbersome route, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
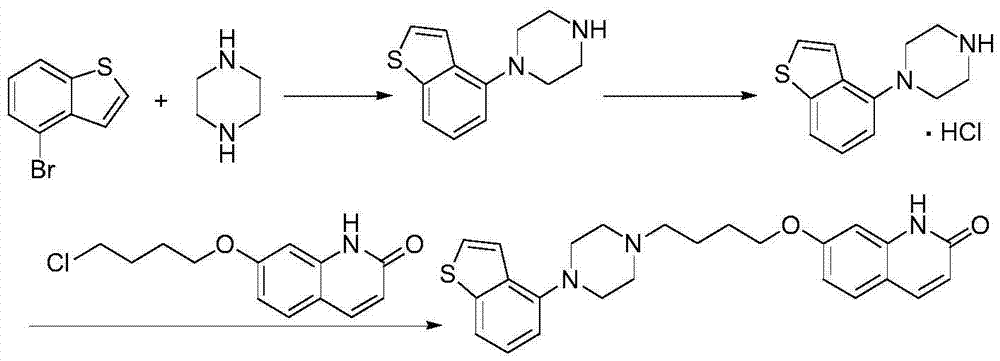
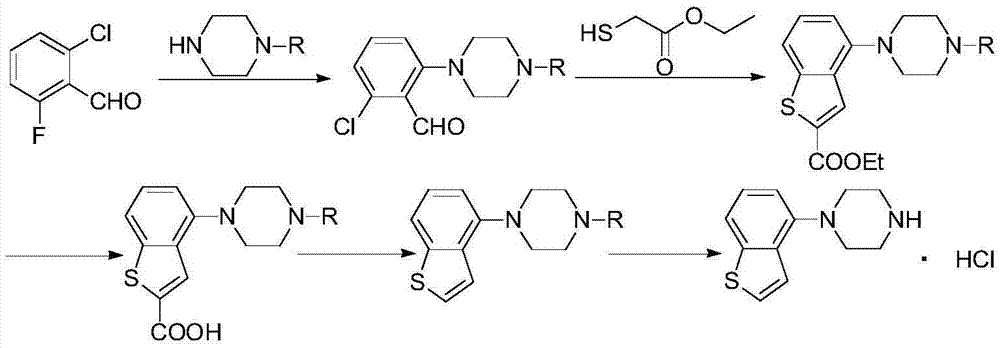
[0059] According to one aspect of the present invention, the present invention provides a compound or salt thereof represented by formula IV,
[0060]
[0061] Wherein, the salt is selected from one of hydrochloride, hydrobromide, sulfonate, methanesulfonate, p-toluenesulfonate, oxalate, phosphate or maleate.
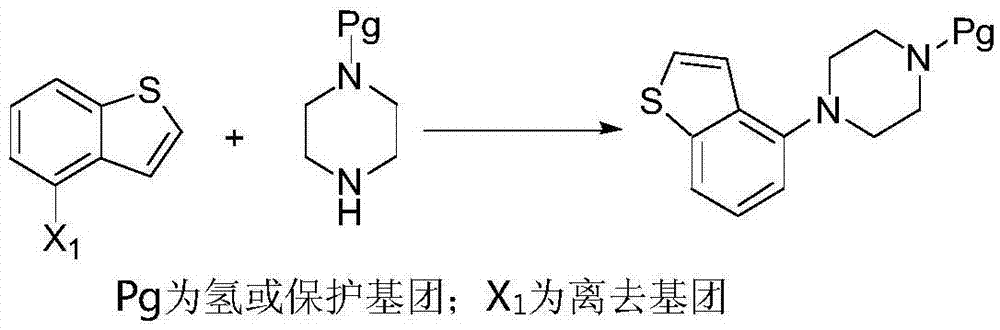
[0062] According to one aspect of the present invention, the present invention provides a method for preparing a compound of formula IV, which comprises deprotecting the compound R of formula III in a protic polar solvent under the catalysis of Blount acid or inorganic base to obtain the compound of formula IV,
[0063]
[0064] Wherein, R in the compound of formula III is an amino protecting group;
[0065]Optionally, the amino protecting group is one of acyl and alkoxycarbonyl;
[0066] Optionally, the acyl group is selected from one of formyl, acetyl or propionyl;
[0067] Optionally, the alkoxycarbonyl group is preferably one of methoxycarbonyl, ethoxycarbon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com