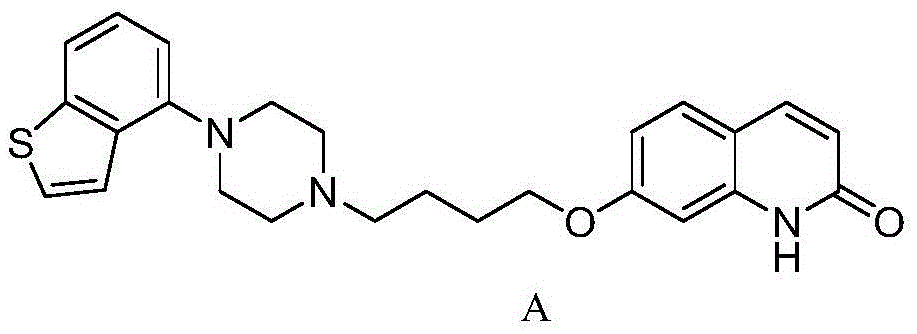
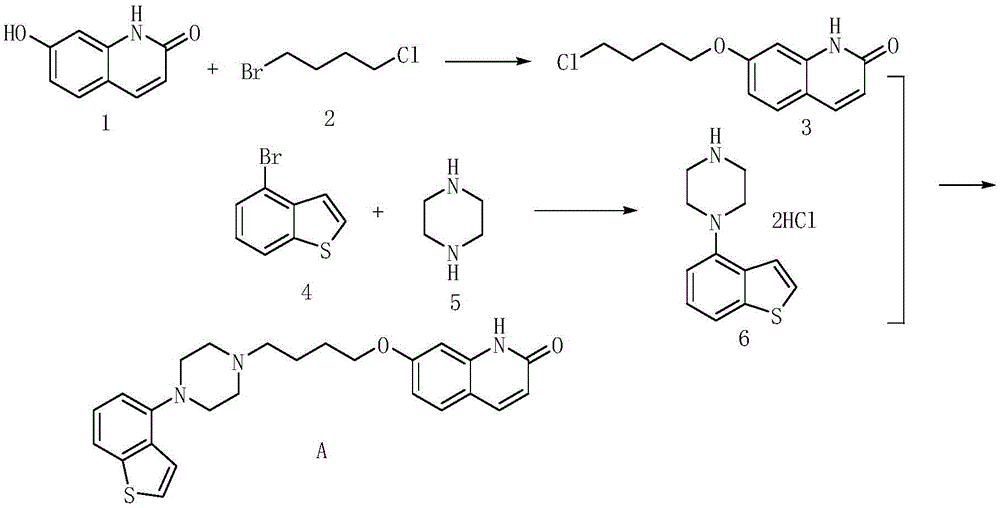
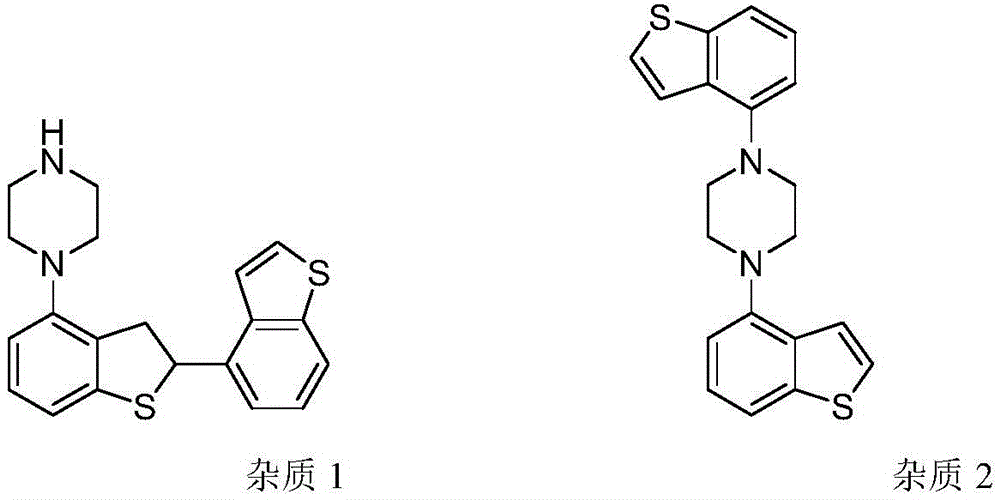
Preparing method for brexpiprazole
A technology for epipiprazole and compound, applied in the field of chemical drug synthesis, can solve the problems of unfavorable industrial preparation of epipiprazole and high cost, and achieve the effects of avoiding metal catalyst, reducing reaction cost and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Preparation of compound shown in formula D
[0054]
[0055] In a 5000mL four-neck round bottom flask, add 400g of the compound shown in formula B (2.5mol), add NMP (nitromethylpyrrolidone) 1600mL, then add 493g of the compound shown in formula C (2.7mol), 489g of DIPEA (N, N-diisopropylethylamine) (3.75mol, 1.5eq), then heat the reaction system to 100°C, and react at this temperature for 12 hours, then cool the reaction system to 5°C, add water and acetone mixture 2000mL (water: acetone = 2:1), and stirred at 5°C for 30 minutes, a light yellow solid precipitated, filtered out the solid, and washed with 500mL of water, and then the obtained solid was air-dried at 50°C to obtain a light yellow solid 805.3 g, yield 97.3%.
[0056] (2) Preparation of compound shown in formula F
[0057]
[0058] In a 10L three-necked flask, add 1.0kg of the compound represented by formula D (3.08mol), 1.7kg of potassium carbonate (12.32mol), 4L of DMF (N,N-dimethylformamide), a...
Embodiment 2
[0076] (1) Preparation of compound shown in formula D
[0077]
[0078] In a 5000mL four-neck round bottom flask, add 400g of the compound shown in Formula B (2.5mol), add 1600mL of dimethyl sulfoxide, and then add 493g of the compound shown in Formula C (2.7mol), 570.5g of DBU (1,8 -diazabicycloundec-7-ene) (3.75mol, 1.5eq), then heated the reaction system to 90°C, and reacted at this temperature for 10 hours, then cooled the reaction system to 5°C, added Mix 2000mL of water and acetone (water: acetone = 2:1), and stir at 0°C for 30 minutes, a light yellow solid precipitates, filter out the solid, wash it with 500mL of water, and blow the resulting solid at 50°C After drying, 787.9 g of light yellow solid was obtained, and the yield was 95.2%.
[0079] (2) Preparation of compound shown in formula F
[0080]
[0081] In a 10L three-necked flask, add 1.0kg of the compound represented by formula D (3.08mol), 492.8g of sodium hydroxide (12.32mol), and 4L of nitrogen methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com