Method for preparing Brexpiprazole with one-pot process
A compound and alcohol solvent technology is applied in the field of preparation of Brexpiprazole to achieve the effects of solving insufficient reaction, simplifying the operation process and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Brexpiprazole
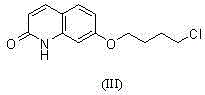
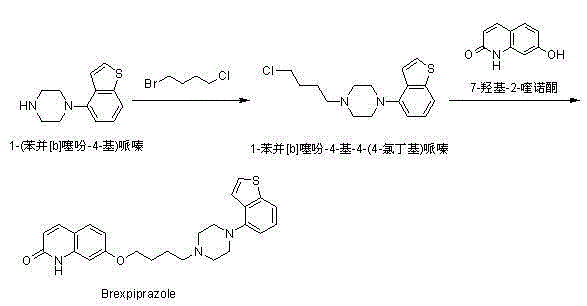
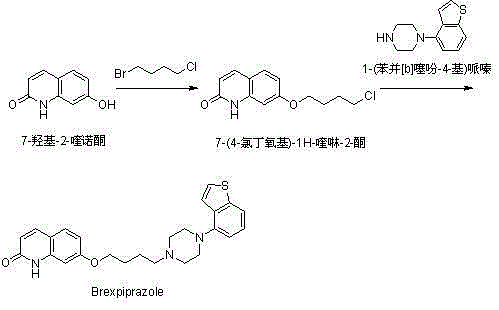
[0046] Add compound (I) 7-hydroxy-2-quinolone (10.0 g, 62 mmol), 120 ml of ethanol and potassium carbonate (19.0 g, 138 mmol) into a 500 ml reaction flask. Compound (II) 1-bromo-4-chlorobutane (12.0 g, 70 mmol) was added under stirring, and the temperature was raised to reflux and stirred for 2 hours. Add 100ml of water and compound (IV) 1-(benzo[b]thiophen-4-yl)piperazine hydrochloride (15.0g, 59mmol), and continue to reflux and stir for 9 hours. Evaporate part of the solvent, lower the temperature to 50°C, add 40ml of ethyl acetate and stir for 0.5 hours, then continue to cool down to below 20°C, filter under reduced pressure, wash the filter cake with 20ml of ethanol three times, and dry it in a blast drying oven at 70°C for 3 hours . 14.5 g of Brexpiprazole (compound V) was obtained with a molar yield of 54%, which was basically the same as the molar yield of 55% in the method described in Route 2.
[0047] 1 HNMR(400MHz,dmso)δ11.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com