Method for separation and determination of brexpiprazole and impurities thereof by liquid chromatography
A technology of ebiprazole and liquid chromatography, which is applied in the field of analytical chemistry, can solve the problems affecting the quality control of ebiprazole and its preparations, and the difficulty in achieving effective separation of ebiprazole, and achieve enhanced retention, high column efficiency, and increased efficiency. The effect of large resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Instruments and Conditions
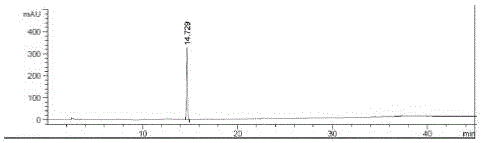
[0055] Agilent 1200 liquid chromatograph and chemical workstation; automatic sample injection; Agilent Eclipse Plus C18 column (5μm, 250×4.6mm) as separation column; UV detector wavelength: 225nm; mobile phase: 0.01mol / L phosphoric acid Potassium dihydrogen solution (adjust the pH value to 3.0 with phosphoric acid solution) as mobile phase A, methanol-acetonitrile (15:85) as mobile phase B, gradient elution; 0 minutes, mobile phase A is 90%, mobile phase B 10%; from 0 minutes to 5 minutes, the mobile phase A is 90%, and the mobile phase B is 10%; from 5 minutes to 35 minutes, the mobile phase A linearly decreases to 20%, and the mobile phase B linearly increases to 80%; 35 minutes Up to 45 minutes, the mobile phase A is 20%, and the mobile phase B is 80%; both mobile phase A and mobile phase B are volume percentages. The column temperature is 25°C, the flow rate is 1.0ml / min, and the injection volume is 20μl.
[0056] Operation method:
...
Embodiment 3
[0062] Determination of impurities in ebiprazole bulk drug.
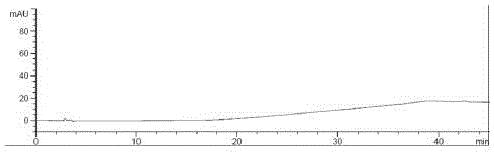
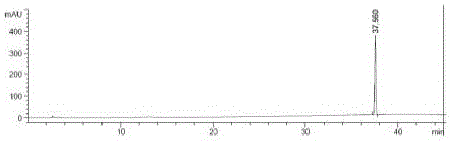
[0063] Take about 10mg of ebiprazole, weigh it accurately, put it in a 50ml measuring bottle, add a diluent (methanol:water=80:20) and sonicate to dissolve and dilute to the mark, shake well, and use it as the test solution; Weigh 10mg of ebiprazole reference substance, put it in a 50ml measuring bottle, add diluent (methanol: water = 80:20) and sonicate to dissolve and dilute to the mark, shake well, accurately measure 1ml, put it in a 100ml measuring bottle, and use Diluent is diluted to scale, shakes up, as contrast solution; Carry out liquid chromatography analysis according to the chromatographic condition of embodiment 1, if impurity peak (except solvent peak) is arranged in the chromatogram of need testing solution, known impurity is multiplied by The peak area after the correction factor shall not be greater than 0.15%, calculated by the external standard method of the main component, and shall not be greate...
Embodiment 4
[0065] Determination of impurities in ebiprazole tablets by liquid chromatography.
[0066] Take an appropriate amount of this product (approximately equivalent to ebiprazole 10mg), put it in a 50ml measuring bottle, add a diluent (methanol: water = 80:20) and ultrasonically treat it to dissolve and dilute to the mark, shake well, and use it as the test solution ; In addition, accurately weigh 10 mg of ebiprazole reference substance, put it in a 50 ml measuring bottle, add diluent (methanol: water = 80: 20) and sonicate to dissolve and dilute to the scale, shake well, and accurately measure 1 ml and place it in 100 ml bottle, dilute to the mark with diluent, shake well, and use it as a contrast solution; in addition, take an appropriate amount of blank adjuvant according to the prescription ratio, and prepare the blank adjuvant test solution according to the same method as the test solution; carry out liquid phase according to the chromatographic conditions of Example 1 For ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com