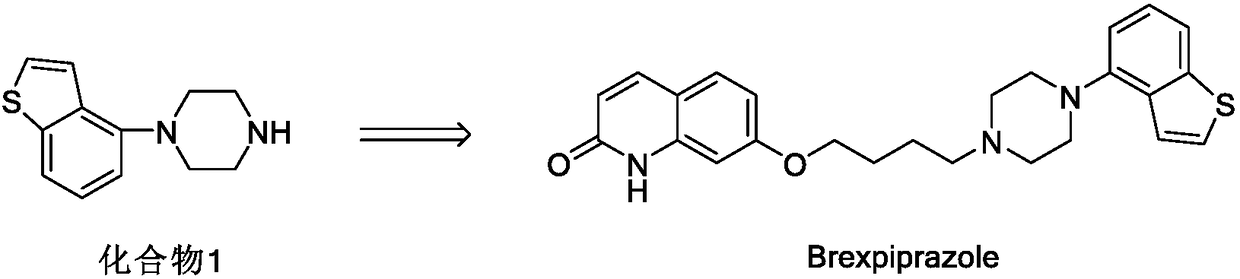
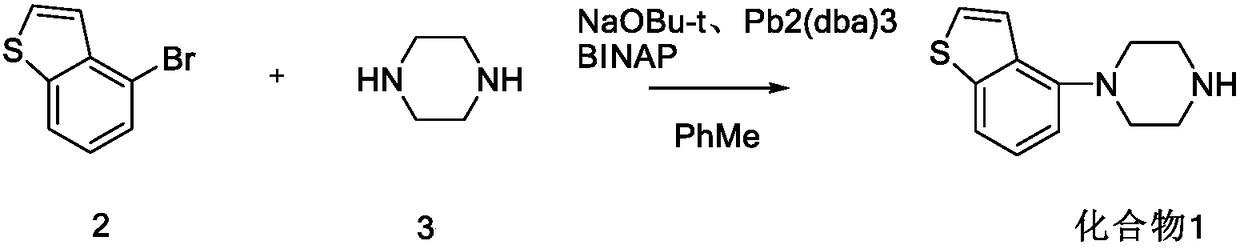
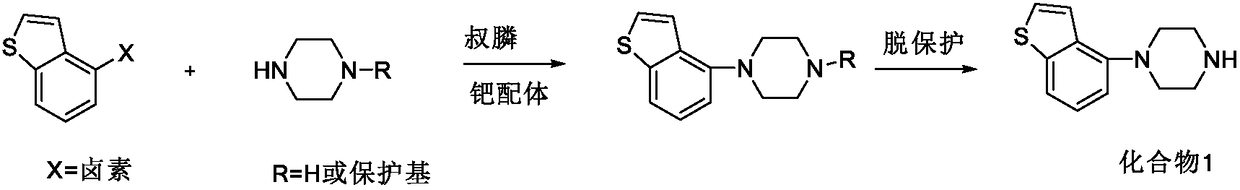
Preparation method of benzothiophene compound intermediate
A technology of compound and thiophene, which is applied in the field of preparation of benzothiophene compound intermediates, can solve the problems of high industrialization cost, unfriendly environment, harsh reaction conditions, etc., and achieve the effects of simple operation, low cost and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 5g 4-bromobenzo[b]thiophene (23.5mmol, 1.0eq), 6g anhydrous piperazine (70mmol, 3.0eq), 0.45g CuI (2.3mmol, 0.1eq), 25mlDMF (5V) into a 100ml three-necked flask , 2.7g L-proline (23mmol, 1.0eq), 3.2g K2CO3 (23.5mmol, 1.0eq). Under the protection of N2, keep warm at 110°C and stir for 10h, drop in 75ml of water, filter out the solid, extract the mother liquor once with 200ml of dichloromethane, concentrate to dryness under reduced pressure, add 75ml of toluene, add about 3ml of concentrated hydrochloric acid dropwise at 0-10°C, A white solid precipitated, stirred at 0-10°C for 1 h, filtered out the solid, and dried at 45°C to obtain 4.8 g of off-white solid with a yield of 80.54%.
[0034] 1H-NMR (DMSO-d6, 400MHz): 9.54 (2H, s), 7.85~7.83 (1H, d, J=8.0Hz), 7.79~7.77 (1H, d, J=8.0Hz), 7.62~7.61 (1H,m),7.42~7.38(1H,t,J=16Hz),7.06~7.01(1H,d,J=8Hz),3.44(4H,s),3.39( 4H,s).MS / ES: m / z:219.09[M+H]+
Embodiment 2
[0036] Add 5g 4-bromobenzo[b]thiophene (23.5mmol, 1.0eq), 6g anhydrous piperazine (70mmol, 3.0eq), 0.45g CuI (2.3mmol, 0.1eq), 25mlDMF (5V) into a 100ml three-necked flask , 0.27g L-proline (2.3mmol, 0.1eq), 3.2g K2CO3 (23.5mmol, 1.0eq), under N2 protection, keep warm at 110°C and stir for 10h, drop into 75ml of water, filter out the solid, and use 200ml of dichloromethane for the mother liquor Methane was extracted once, 3ml of concentrated hydrochloric acid was added dropwise at 0-10°C, a white solid was precipitated in the system, and 3.0g of off-white solid was obtained by column chromatography, with a yield of 50.34%.
[0037] 1H-NMR (DMSO-d6, 400MHz): 9.54 (2H, s), 7.85~7.83 (1H, d, J=8.0Hz), 7.79~7.77 (1H, d, J=8.0Hz), 7.62~7.61 (1H,m),7.42~7.38(1H,t,J=16Hz),7.06~7.01(1H,d,J=8Hz),3.44(4H,s),3.39( 4H,s).MS / ES: m / z:219.09[M+H]+
Embodiment 3
[0039] Add 5g 4-bromobenzo[b]thiophene (23.5mmol, 1.0eq), 6g anhydrous piperazine (70mmol, 3.0eq), 0.45g CuI (2.3mmol, 0.1eq), 25mlDMF (5V) into a 100ml three-necked flask , 27g L-proline (230mmol, 10.0eq), 3.2g K2CO3 (23.5mmol, 1.0eq), under N2 protection, keep warm at 110°C and stir for 10h, drop into 75ml of water, filter out the solid, and extract the mother liquor with 200ml of dichloromethane Once, concentrate to dryness under reduced pressure, add 75ml of toluene, add about 3ml of concentrated hydrochloric acid dropwise at 0-10°C, a white solid precipitates in the system, stir at 0-10°C for 1h, filter out the solid, and dry at 45°C to obtain 3.5g of off-white solid , yield 58.73%.
[0040]1H-NMR (DMSO-d6, 400MHz): 9.54 (2H, s), 7.85~7.83 (1H, d, J=8.0Hz), 7.79~7.77 (1H, d, J=8.0Hz), 7.62~7.61 (1H,m),7.42~7.38(1H,t,J=16Hz),7.06~7.01(1H,d,J=8Hz),3.44(4H,s),3.39( 4H,s).MS / ES: m / z:219.09[M+H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com