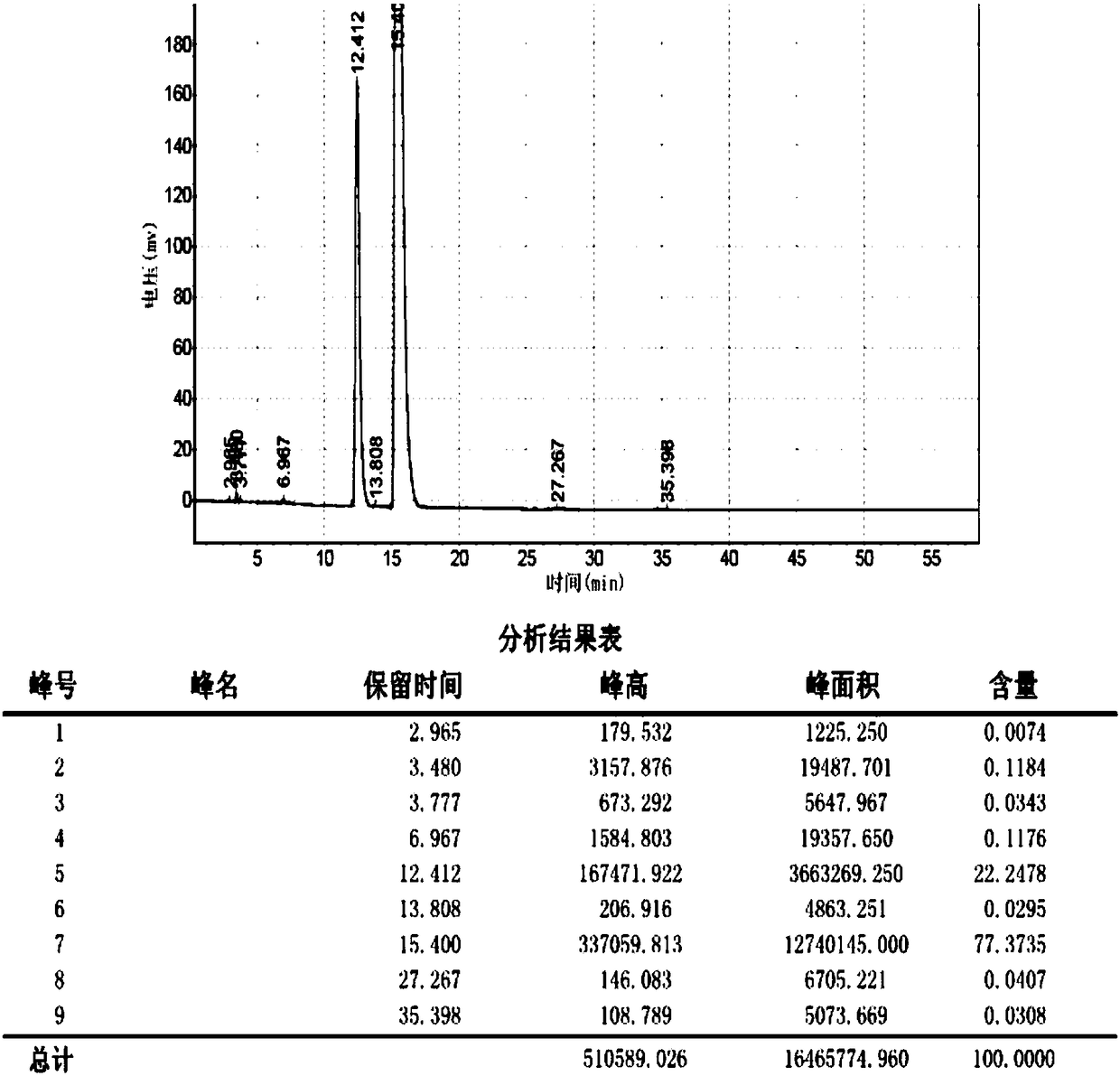
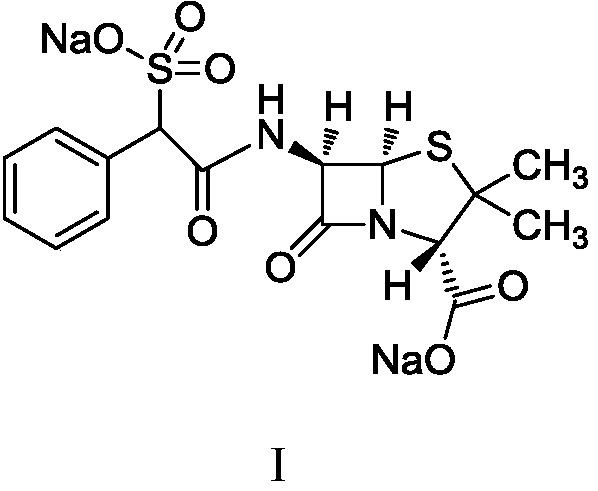
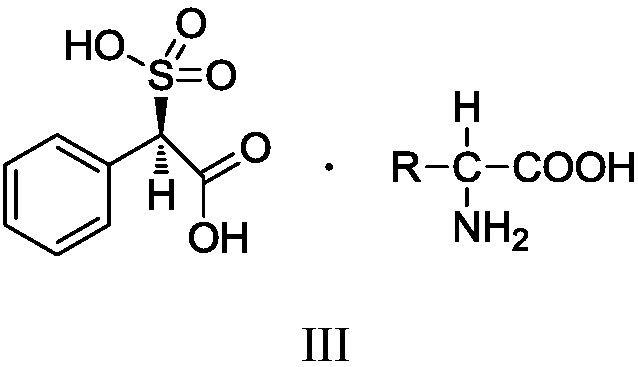
Preparation method of sulbenicillin sodium
A technology of sulfbenicillin sodium and sodium isooctanoate, which is applied in the field of preparation of antibiotic drug sulfbenicillin sodium, can solve the problems of impurity generation and yield reduction, unfavorable control of the ratio of dextrorotary body, and increase of operation steps, etc., and the method is simple , reducing the amount of hydrolyzed impurities, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) In 100ml of water, add 15.15g of L-histidine, add 20g of D,L-sulfophenylacetic acid, heat up to 45°C, stir and react for 2 hours; slowly cool down to 0°C to 10°C, stir and crystallize for 2 hour; filter, and wash the filter cake with an appropriate amount of cold ethanol to obtain 21 g of D(-)-sulfophenylacetic acid histidine salt, with a yield of 61.0%;
[0060] (2) Add D(-)-sulfophenylacetic acid histidine salt obtained in the previous step to 100ml of ethanol, adjust the temperature of the feed liquid to 20°C, add 6.5g of concentrated hydrochloric acid dropwise, stir for 2 hours, filter, and distill the filtrate under reduced pressure 12 g of D(-)-sulfophenylacetic acid was obtained with a yield of 98.4%;
[0061] (3) Mix D(-)-sulfophenylacetic acid (10.07g) with D,L-sulfophenylacetic acid (7.93g) at a mass ratio of 1.27:1 to obtain D(-)-sulfophenylacetic acid: L(+)-sulfophenylacetic acid=78%: 22%, obtain the mixture based on D(-)-sulfophenylacetic acid mixed in...
Embodiment 2
[0067] (1) In 100ml of water, add 14.2g of L-lysine and 20g of D,L-sulfophenylacetic acid, raise the temperature to 40°C, and stir for 2.5 hours. Slowly lower the temperature to 0°C-5°C, stir and crystallize for 2 hours. Filter and wash the filter cake with appropriate amount of cold ethanol. 18.7 g of D(-)-sulfophenylacetic acid lysine salt was obtained, with a yield of 55.8%;
[0068] (2) Add D(-)-sulfophenylacetic acid lysine salt obtained in the previous step to 90ml of ethanol, adjust the feed liquid temperature to 18°C, add 6.0g of concentrated hydrochloric acid dropwise, stir for 3 hours, filter, and distill the filtrate under reduced pressure 11.1 g of D(-)-sulfophenylacetic acid was obtained. Yield 99.5%;
[0069] (3) Mix D(-)-sulfophenylacetic acid (11.0g) with D,L-sulfophenylacetic acid (5.5g) in a ratio of 2:1 to obtain D(-)-sulfophenylacetic acid: L (+)-sulfophenylacetic acid=80%: 20%, obtain the mixture based on D(-)-sulfophenylacetic acid mixed in proportion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com