Methods for preparing brexpiprazole intermediate and brexpiprazole with cheap metal copper
A technology for epipiprazole and intermediates, which is applied in the field of synthesis of epipiprazole bulk drugs, can solve the problems of high difficulty, high relevant requirements, and increased reaction costs, and achieves low environmental pollution, high economic benefits, and reduced The effect of reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
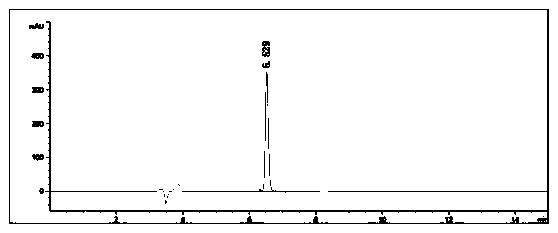
[0079] Example 1: Under nitrogen protection, 4-bromobenzo[b]thiophene (10.7g, 0.05mol), piperazine (4.3g, 0.05mol), cuprous oxide (0.36g, 0.0025mol) were added to a 500mL single-necked bottle ), potassium hydroxide (4.2g, 0.075mol), 1,10-phenanthroline (1.8g, 0.01mol) and N,N'-dimethylformamide (30mL), stirred at 100°C for 24 hours, and the reaction was completed Finally, spin the reaction solution to dry the solvent, add water to the obtained solid and extract it with ethyl acetate, combine the organic phases and spin dry, dissolve the obtained solid in methanol, add 2M hydrochloric acid methanol solution dropwise to adjust the pH to 4, and the target product becomes a salt The precipitated salt was washed with acetone, suction filtered to obtain a white solid, and dried to obtain 9.0 g of 4-piperazinylbenzothiophene hydrochloride, with a yield of 82.6% and a purity of 99.6%. The data characterization of the 4-piperazinylbenzothiophene hydrochloride prepared in embodiment 1: ...
Embodiment 2
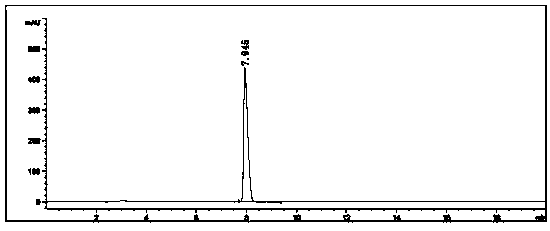
[0082] Example 2: Under nitrogen protection, 4-bromobenzo[b]thiophene (10.7g, 0.05mol), piperazine (21.5g, 0.25mol), cuprous oxide (0.72g, 0.005mol) were added to a 500mL single-necked bottle ), potassium hydroxide (4.2g, 0.075mol), 1,10-phenanthroline (1.8g, 0.01mol) and N,N'-dimethylformamide (30mL), stirred at 100°C for 24 hours, and the reaction was completed Finally, spin the reaction solution to dry the solvent, add water to the obtained solid and extract it with ethyl acetate, combine the organic phases and spin dry, dissolve the obtained solid in methanol, add 2M hydrochloric acid methanol solution dropwise to adjust the pH to 4, and the target product becomes a salt The precipitated salt was washed with acetone, suction filtered to obtain a white solid, and dried to obtain 9.4 g of 4-piperazinylbenzothiophene hydrochloride, with a yield of 86.2% and a purity of 99.4%.
Embodiment 3
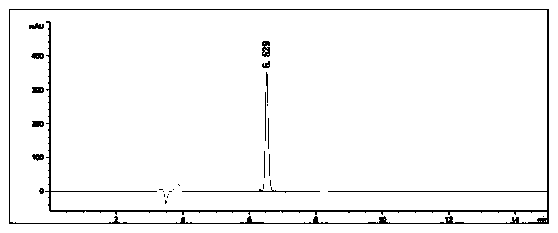
[0083] Example 3: Under nitrogen protection, 4-bromobenzo[b]thiophene (10.7g, 0.05mol), piperazine (43.1g, 0.5mol), cuprous oxide (2.15g, 0.015mol) were added to a 500mL single-necked bottle ), potassium hydroxide (4.2g, 0.075mol), 1,10-phenanthroline (1.8g, 0.01mol) and N,N'-dimethylformamide (30mL), stirred at 100°C for 24 hours, and the reaction was completed Finally, spin the reaction solution to dry the solvent, add water to the obtained solid and extract it with ethyl acetate, combine the organic phases and spin dry, dissolve the obtained solid in methanol, add 2M hydrochloric acid methanol solution dropwise to adjust the pH to 4, and the target product becomes a salt The precipitated salt was washed with acetone, suction filtered to obtain a white solid, and dried to obtain 9.5 g of 4-piperazinylbenzothiophene hydrochloride, with a yield of 87.2% and a purity of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com