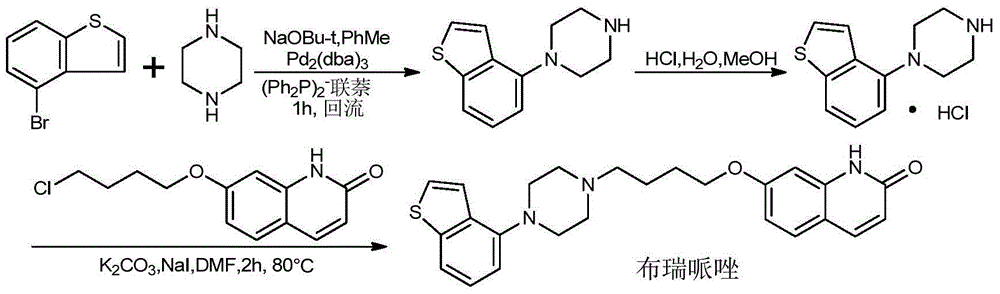
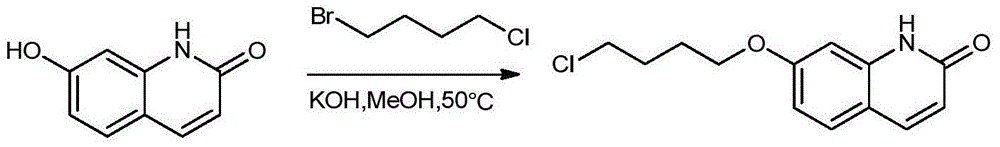
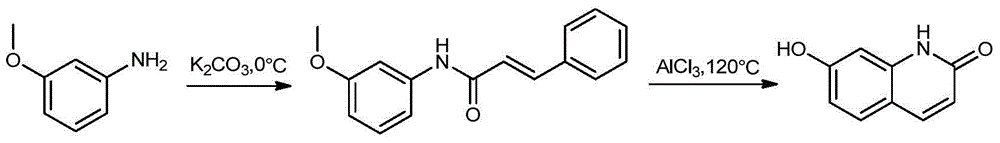
Preparation method of novel brexpiprazole, aripiprazole and salts thereof
A technology of brepiprazole and aripiprazole, applied in the field of medicinal chemistry, can solve the problems of poor selectivity and affecting the purity and yield of the final product breiprazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Embodiment 1: the preparation of 7-chlorobutoxy coumarin
[0189]
[0190] 7-hydroxycoumarin (500mg, 3.09mmol), potassium carbonate (640mg, 4.64mmol), 1-bromo-4-chlorobutane (426μl, 3.71mmol) and 10ml of ethanol were dropped into the reaction flask successively, and then heated to reflux 12h. After removing ethanol, add water to dissolve potassium carbonate, filter with suction, and wash the filter cake twice with 20ml of petroleum ether. After drying, 606 mg of white product was obtained.
Embodiment 2
[0191] Embodiment 2: the preparation of compound IIa-1
[0192]
[0193] Add 7-chlorobutoxycoumarin (255mg, 1mmol), potassium carbonate (276, 2mmol), sodium iodide (150, 1mmol), 1-(benzothiophen-4-yl)piperone into the reaction flask successively Oxyzine (218mg, 1mmol) and 10ml of acetonitrile, then heated to reflux for 24h. After the acetonitrile was removed, water was added to dissolve potassium carbonate, and suction filtered. After the filter cake was slurried with 10 ml of acetonitrile, suction filtered and dried to obtain product IIa-1 (380 mg).
Embodiment 3
[0194] Embodiment 3: the preparation of brepiprazole
[0195] Compound IIa-1 was dissolved in ammonia-methanol solution, pressurized, and heated at 150°C. After the reaction, spin-dried, washed once with water and once with saturated aqueous sodium carbonate solution, and dried to obtain breiprazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com