Brexpiprazole-containing freeze-dried oral preparation and preparation method thereof
A technology for oral preparation and epipiprazole, which is applied in the field of freeze-dried oral preparation containing epipiprazole and its preparation, can solve problems such as blank, and achieve the effects of low water content, improved bioavailability and light weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
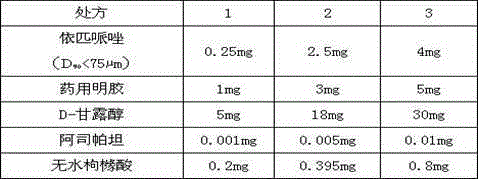
[0042] This example provides three kinds of freeze-dried oral preparations containing ebiprazole, and their prescriptions and contents are shown in Table 1 below.
[0043]
[0044] Table 1
[0045] It is easy to understand that the above-mentioned excipients of the freeze-dried preparation can also be replaced by other similar excipients, and the dosage of each excipient should be adjusted accordingly according to the actual usage, so as to meet the medicinal dosage requirements of the excipients.
[0046]In the above prescription, aspartame or anhydrous citric acid can also be used alone as a flavoring agent, and the dosage is 0.001-0.81 mg. Preferably, aspartame and anhydrous citric acid are used as flavoring agents at the same time to adjust sweetness and sourness.
Embodiment 2
[0048] This embodiment provides a method for preparing the lyophilized oral preparation containing ebiprazole described in Example 1, comprising the following steps:
[0049] A. Measure 1400ml of purified water into a container, add the prescribed amount of D-mannitol, aspartame and anhydrous citric acid, stir to dissolve and then add medicinal gelatin; after the medicinal gelatin is stirred and dissolved, add The pulverized ebiprazole raw material is stirred to form a suspension solution, and water is added to the prescribed amount according to the weight of the dosing solution, and then fully sheared and stirred, and stirred for more than 30 minutes to obtain a stable suspension solution.
[0050] B. Pour the stable suspension solution in step A into a cold aluminum foil model in a 0.3ml single-dose package, use liquid nitrogen (-80°C) spray technology to freeze quickly, and transfer it to a freeze dryer for sublimation drying. Drying and constant temperature drying, so that...
Embodiment 3
[0057] This embodiment evaluates the quality of three lyophilized oral preparations containing ebiprazole in Example 1, including:
[0058] (1) Evaluation of disintegration: put the product on a glass plane, add 1 drop of water (about 0.02ml) to the surface of the product at a distance of 0.5cm from the tablet and time it accurately; it is required to measure 10 tablets, and the standard limit of disintegration time for each tablet For no more than 10 seconds.
[0059] (2) Taste evaluation: 10 volunteers evaluated the taste, put the product on the tip of their tongues, observed the disintegration situation and recorded the time, whether there is a sandy feeling after complete disintegration, whether the sweet and sour taste is good, etc.
[0060] (3) Character evaluation: The surface of the product is required to be smooth; the packaging is completely stripped of the film.
[0061] (4) Moisture evaluation: The Fischer method is used to measure the moisture, and the moisture c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com