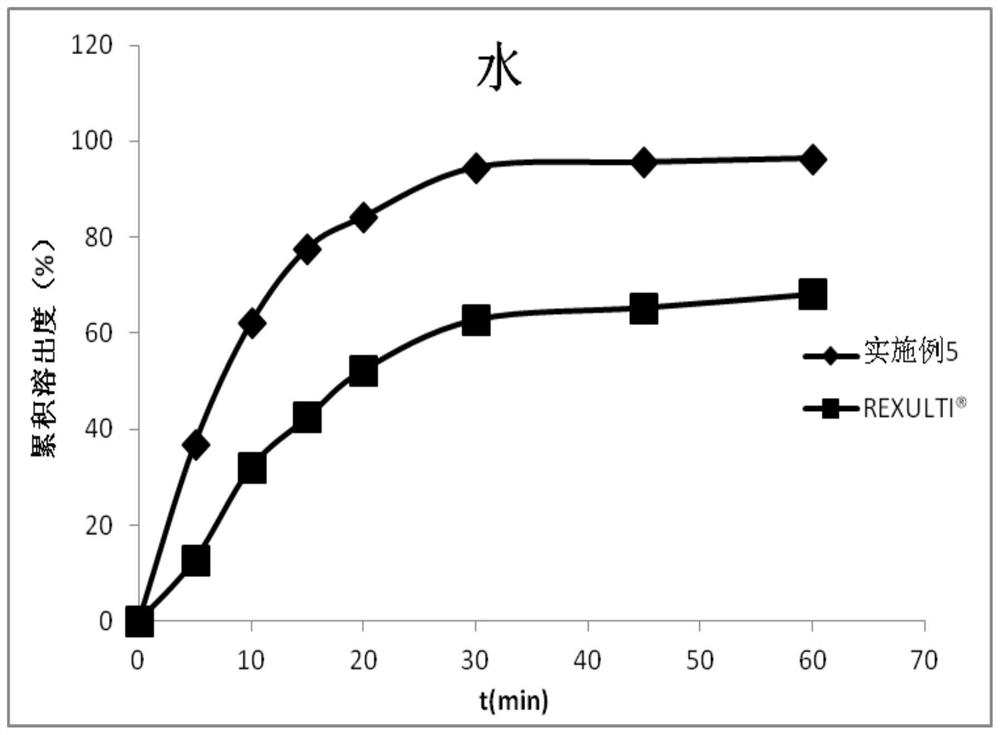
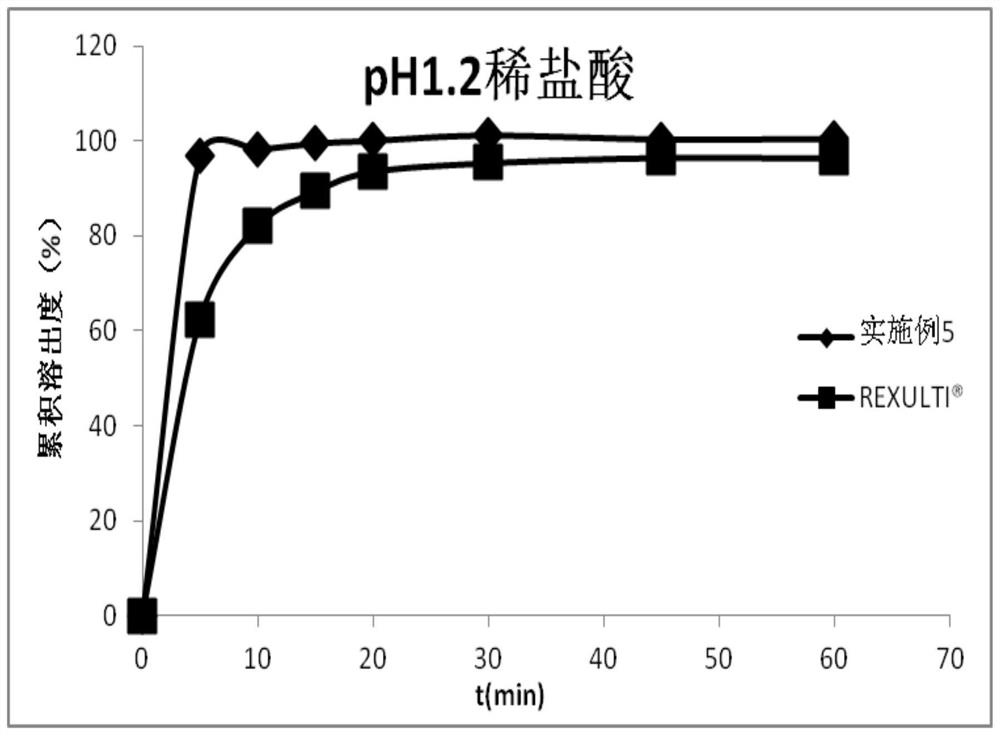
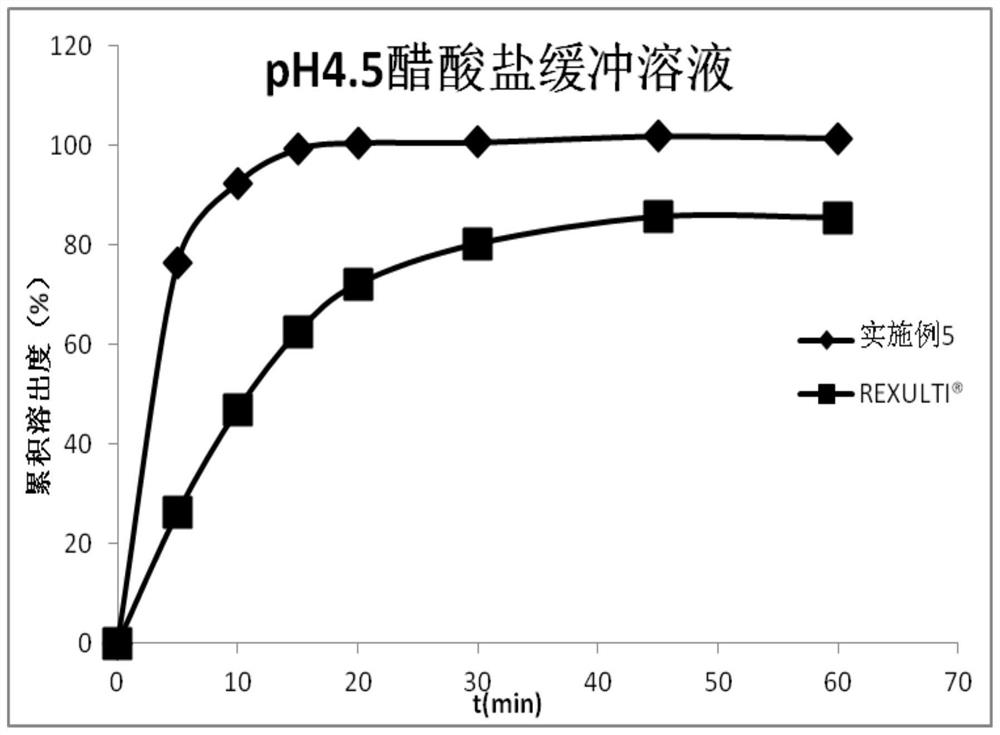
Pharmaceutical composition containing brexpiprazole and amphiphilic polymer as well as preparation method and application of pharmaceutical composition
The technology of an amphiphilic polymer and bripiprazole is applied in the field of pharmaceutical compositions comprising bripiprazole and an amphiphilic polymer and the preparation thereof, and can solve the problem of poor medication compliance of patients, single medication compliance, use of Inconvenience and other problems, to achieve the effect of reducing clinical care costs, improving bioavailability, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of a pharmaceutical composition comprising bripiprazole and an amphiphilic polymer, comprising the following steps:
[0043] S1. Grinding brepiprazole until the average particle size is less than 100 μm;
[0044] S2. Mix the prescribed amount of brepiprazole with the amphiphilic polymer and dissolve it in the solvent, then add purified water to redissolve after rotary evaporation, and obtain the brepiprazole-amphiphilic polymer inclusion compound after filtration ;
[0045] S3. Add a predetermined amount of film-forming agent, plasticizer, stabilizer, flavoring agent and opacifier to the bripiprazole-amphiphilic polymer clathrate obtained in step S2, and stir thoroughly to obtain a milky solution;
[0046] S4. Defoaming the milky solution obtained in step S3, coating it, drying it, cutting it and dividing it to obtain a pharmaceutical composition containing bripiprazole and an amphiphilic polymer.
[0047] In s...
Embodiment 1
[0051] This embodiment provides a pharmaceutical composition comprising breiprazole and amphiphilic polymer α-tocopheryl polyethylene glycol succinate (TPGS), the breiprazole is encapsulated in the lipophilic core of TPGS , the formation of bripiprazole-TPGS inclusion complex.
[0052] Wherein, the preparation method of brepiprazole-TPGS clathrate comprises the following steps:
[0053] S1. Grinding, sieving or jet milling is used to pulverize the bripiprazole, with the average particle size controlled below 100 μm, and set aside;
[0054] S2. Mix the prescribed amount of breiprazole and α-tocopheryl polyethylene glycol succinate (TPGS) and dissolve them in a predetermined amount of ethanol, then place them in a rotary evaporator, and evaporate to dryness at 50°C ethanol to obtain a thin film; then add a predetermined amount of purified water to the above-mentioned rotary evaporator, stir at 50° C. at a speed of 200 rpm for 1.5 h, and then filter to obtain the buripiprazole-T...
Embodiment 2~4 and comparative example 1
[0056] Embodiments 2 to 4 respectively provide a preparation method of brepiprazole-TPGS clathrate. Compared with Example 1, the difference is that the consumption and proportion of breiprazole and TPGS are changed. The corresponding raw material consumption of example and comparative example is as shown in table 1.
[0057] The consumption and proportioning of each raw material in Table 1 embodiment 1~4 and comparative example 1
[0058]
[0059] The encapsulation efficiency of the bripiprazole-TPGS inclusion compound prepared in Examples 1-4 and Comparative Example 1 was tested, and the results are shown in Table 2.
[0060] The encapsulation efficiency measurement result of table 2 embodiment 1~4 and comparative example 1
[0061] Example / Comparative example Encapsulation rate (%) Example 1 79.4±1.02 Example 2 82.4±1.02 Example 3 87.6±1.02 Example 4 86.2±0.98 Comparative example 1 76.3±1.02
[0062] It can be seen from Tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com