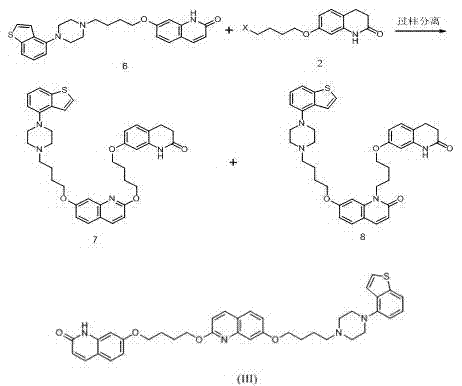
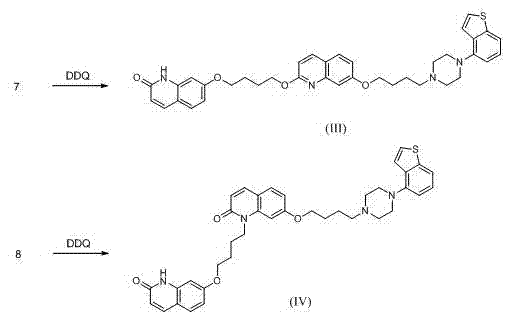
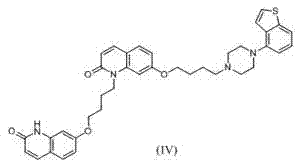
Brexpiprazole impurity compound and preparation method thereof
A technology of ebiprazole and compounds, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 7-(4-(2-oxo-1,2,3,4-tetrahydroquinolin-7-oxyl)butoxy)-3,4-dihydroquinolin-2(1H)-one ( Compound 3) Preparation
[0043] Add 2.09g of 7-hydroxy-3,4-dihydroquinolin-2(1H)-one and 485mg of lithium hydroxide monohydrate to a mixed solvent of 40ml of N-methylpyrrolidone and 4ml of water at 40°C and stir for 2 hours After cooling down to room temperature, add 4.0g of 7-(4-iodobutoxy)-3,4-dihydroquinolin-2(1H)-one and stir until the reaction is basically completed, add 50ml of methanol to crystallize, and filter with suction. Drying yielded 3.2 g of product.
Embodiment 2
[0045] Preparation of 7-(4-(2-oxo-1,2-dihydroquinolin-7-oxyl)butoxy)quinolin-2(1H)-one (compound I)
[0046] Add 5.9g DDQ to 40ml dimethyl sulfoxide and stir at 40°C, and take the 7-(4-(2-oxo-1,2,3,4-tetrahydroquinoline- 2.0g of 7-oxyl)butoxy)-3,4-dihydroquinolin-2(1H)-one, dissolved in 150ml dimethyl sulfoxide by heating, then added dropwise to the dimethyl sulfoxide solution of DDQ, kept warm Reacted for 4 hours. After the reaction was completed, the temperature was lowered, and a total of 80 ml of sodium sulfite and sodium bicarbonate aqueous solution was added dropwise. A solid precipitated, which was suction filtered, washed with water, and dried to obtain 1.3 g of the title compound.
[0047] 1 H-NMR (DMSO-d 6 ) δ ppm:
[0048] 1.197 (4H, brs),4.084 (4H, brs), 6.275~6.299 (2H, d), 6.784~6.803 (4H,m), 7.538~ 7.561 (2H, d), 7.785~7.808 (2H,d), 11.565 (1H, s).
Embodiment 3
[0050] Preparation of 5-(4-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)butoxy)quinolin-2(1H)-one (compound II)
[0051]5.0 g of 5-(4-chlorobutoxy)-quinolin-2(1H)-one and 5.5 g of 4-bromobenzo[b]thiophene hydrochloride, 3.55 g of potassium carbonate and 2.04 g of sodium bromide were added Put it into 40ml of N-methylpyrrolidone, raise the temperature to 70-80°C and react for 3-5 hours, after the reaction is completed, cool down to room temperature, add 120ml of water, a white solid precipitates, filter with suction, wash the filter cake twice with water, and dry under reduced pressure 7.52 g of the title compound were obtained.
[0052] 1 H-NMR (DMSO-d 6 ) δ ppm:
[0053] 1.653~1.726 (2H, qunt), 1.767~1.863 (2H, qunt), 2.446~2.482 (2H,t), 2.624(4H, brs), 3.064 (4H, brs), 4.124~4.155 (2H, t) , 6.426~6.450 (1H, d), 6.727~6.747 (1H, d), 6.862~6.881 (1H, d), 6.881~6.899 (1H, d), 7.253~7.292 (1H, t), 7.388~7.402 (1H , d), 7.382~7.423 (1H, t), 7.600~7.620 (1H, d), 7.680~7.694(1H, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com