Analysis and detection method for rivaroxaban intermediate
A detection method, the technology of rivaroxaban, which is applied in the field of high-performance liquid chromatography analysis, can solve the problems affecting the quality of the final product rivaroxaban, and achieve stable and reliable results, good repeatability and durability, and short analysis time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Instrument and conditions: Agilent1200 liquid chromatography system, chromatographic column: Agilent SB-C18 (4.6×150mm, 5μm), detection wavelength 245nm, column temperature 35°C, flow rate 1.0ml / min, mobile phase A is adjusted pH with triethylamine =3 of 0.5% perchloric acid aqueous solution, the mobile phase B is methanol, and the gradient elution settings are as follows:
[0026]
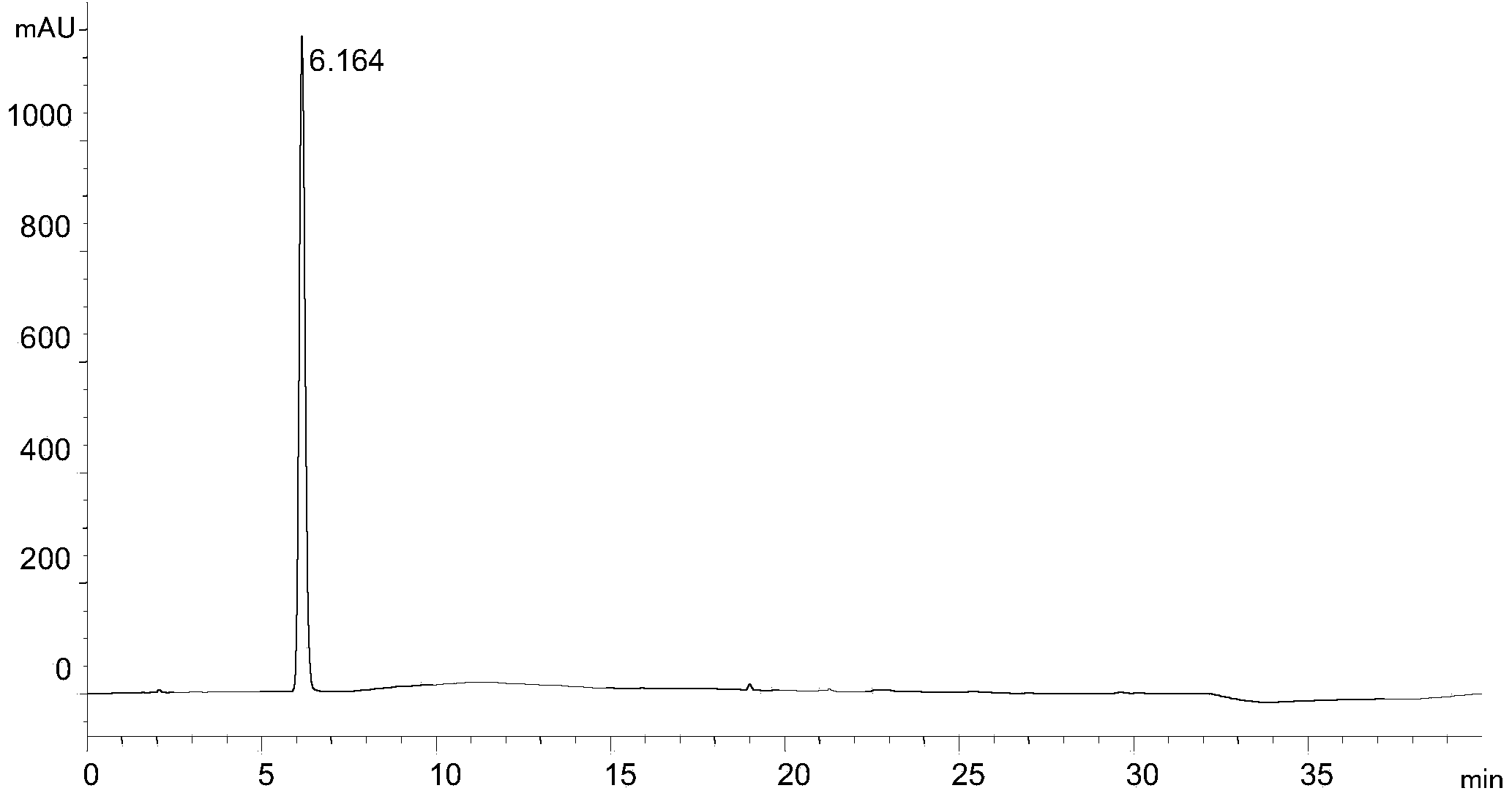
[0027] Experimental procedure: Dissolve the rivaroxaban intermediate in 40% (volume ratio) methanol aqueous solution and quantitatively dilute it to make a solution containing 1.0mg of rivaroxaban intermediate per 1ml, as the test solution, and accurately measure the 5 μ l of the test solution was injected into the liquid chromatograph, and the high-performance liquid chromatography was analyzed according to the above conditions, and the chromatogram was recorded. The results are shown in the attached figure 1 .
[0028] attached figure 1 It shows that under the chromatographic conditi...
Embodiment 2
[0030] Instrument and conditions: Agilent1200 liquid chromatography system, chromatographic column: Agilent SB-C18 (4.6×150mm, 5μm), detection wavelength 245nm, column temperature 35°C, flow rate 1.1ml / min, mobile phase A is adjusted pH with triethylamine =3.5 of 0.6% perchloric acid aqueous solution, the mobile phase B is methanol, and the gradient elution settings are as follows:
[0031]
[0032]
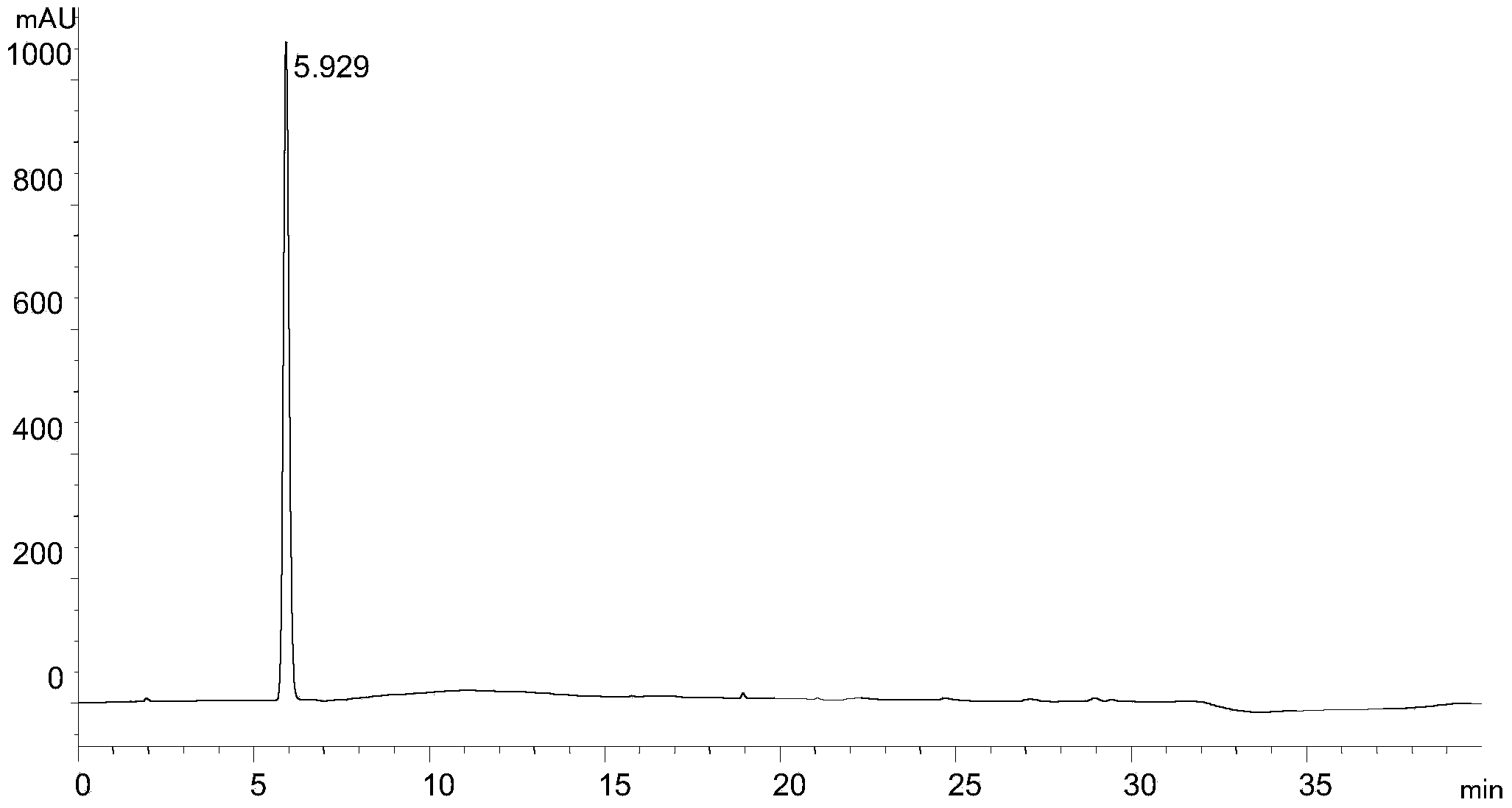
[0033] Experimental procedure: Dissolve the rivaroxaban intermediate in 40% (volume ratio) methanol aqueous solution and quantitatively dilute it to make a solution containing 1.0mg of rivaroxaban intermediate per 1ml, as the test solution, and accurately measure the 5 μ l of the test solution was injected into the liquid chromatograph, and the high-performance liquid chromatography was analyzed according to the above conditions, and the chromatogram was recorded. The results are shown in the attached figure 2 .
[0034] attached figure 2 It shows that under the chromat...
Embodiment 3
[0036] Instrument and conditions: Agilent1200 liquid chromatography system, chromatographic column: Agilent SB-C18 (4.6×150mm, 5μm), detection wavelength 245nm, column temperature 35°C, flow rate 0.7ml / min, mobile phase A is adjusted pH with triethylamine =2.5 of 0.4% perchloric acid aqueous solution, the mobile phase B is methanol, and the gradient elution settings are as follows:
[0037]
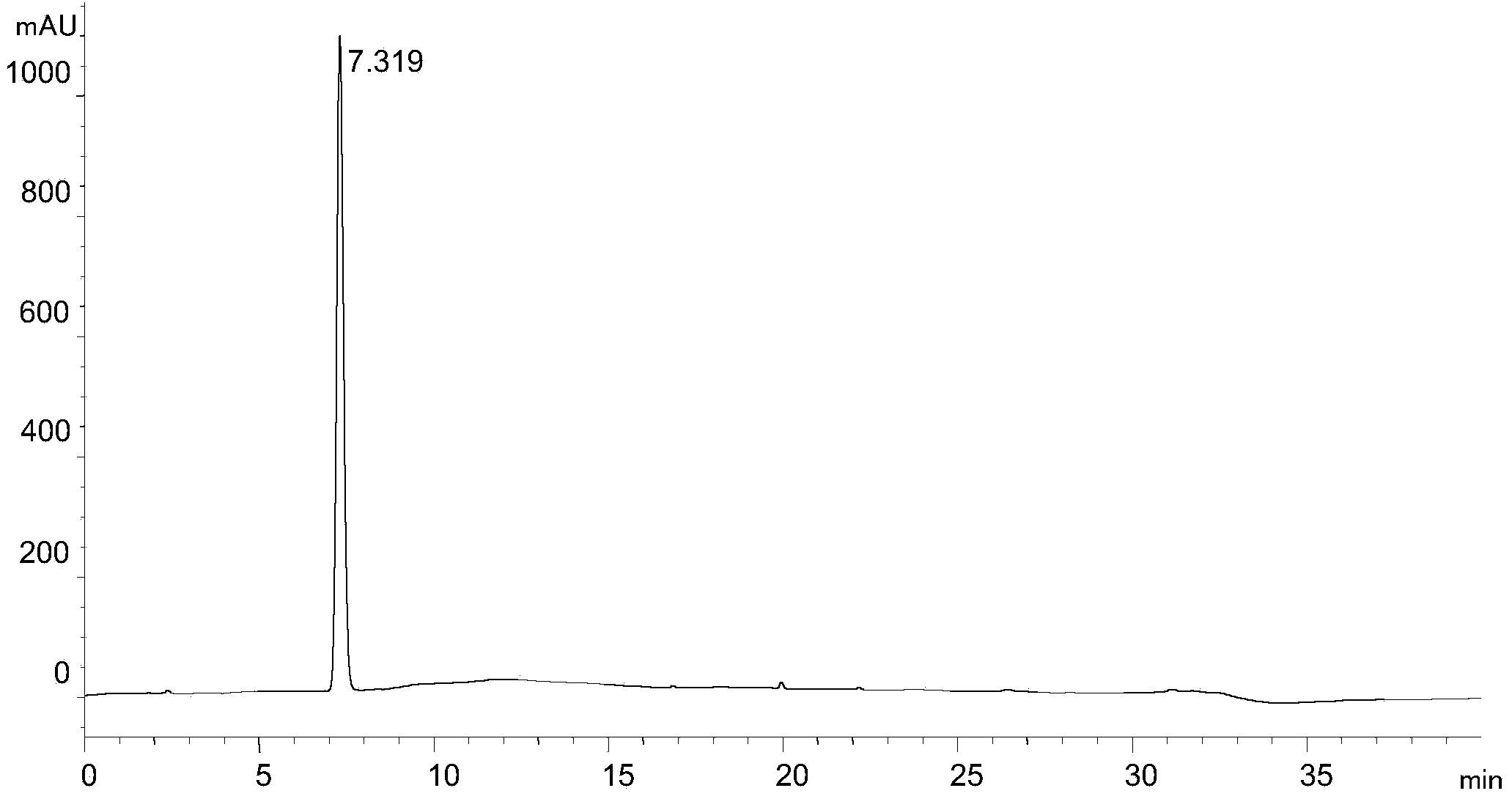
[0038] Experimental procedure: Dissolve the rivaroxaban intermediate in 40% (volume ratio) methanol aqueous solution and quantitatively dilute it to make a solution containing 1.0mg of rivaroxaban intermediate per 1ml, as the test solution, and accurately measure the 5 μ l of the test solution was injected into the liquid chromatograph, and the high-performance liquid chromatography was analyzed according to the above conditions, and the chromatogram was recorded. The results are shown in the attached image 3 .
[0039] attached image 3 It shows that under the chromatographic condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com