Quality control method of Dayuan decoction composition
A quality control method and composition technology, which are applied in important analysis fields, can solve the problems of few indicators, single quality control, as many as the original drink composition, etc., and achieve the effects of objective conclusion, convenient operation and control of preparation quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1. The preparation of Dayuan drink composition
[0092] The first Dayuan drink composition: Weigh the following decoction pieces by weight: 6g betel nut, 3g magnolia officinalis, 1.5g grass nut, 3g anemarrhena, 3g white peony root, 3g skullcap, 1g licorice, and place them in a 2L decoction casserole , add 400ml of water, soak for 30 minutes, cover, place the casserole on an electric heating plate, heat it to a boil (about 13 minutes) on a strong fire (voltage value of 220V), keep it on a slow fire (voltage value of about 170V) until the medicinal liquid is about 160ml (about 40 minutes), use two layers of medical gauze to filter out the medicine residue, let it cool, and get it.
[0093] The second Dayuan drink composition: take the following decoction pieces by weight: 1200g betel nut, 600g magnolia bark, 300g grass nut, 600g anemarrhena, 600g white peony root, 600g scutellaria baicalensis, 300g licorice; add 8 times the amount of water, decoct Boil for 1.0...
Embodiment 2
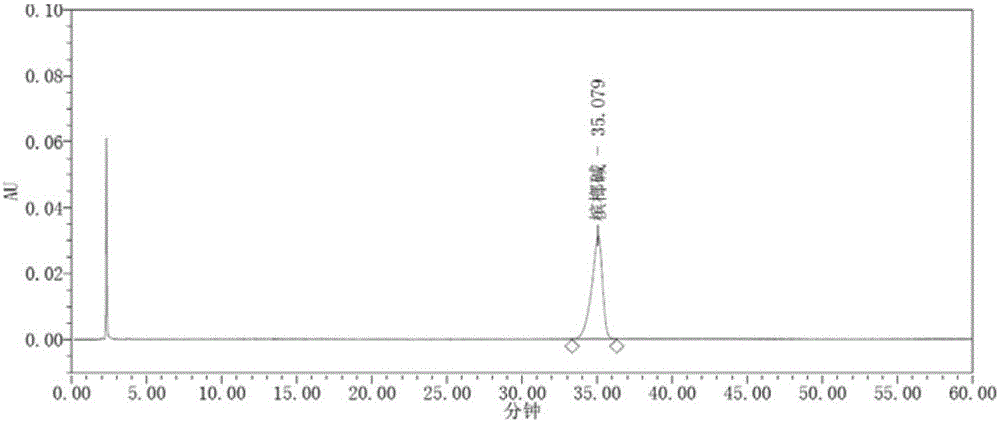
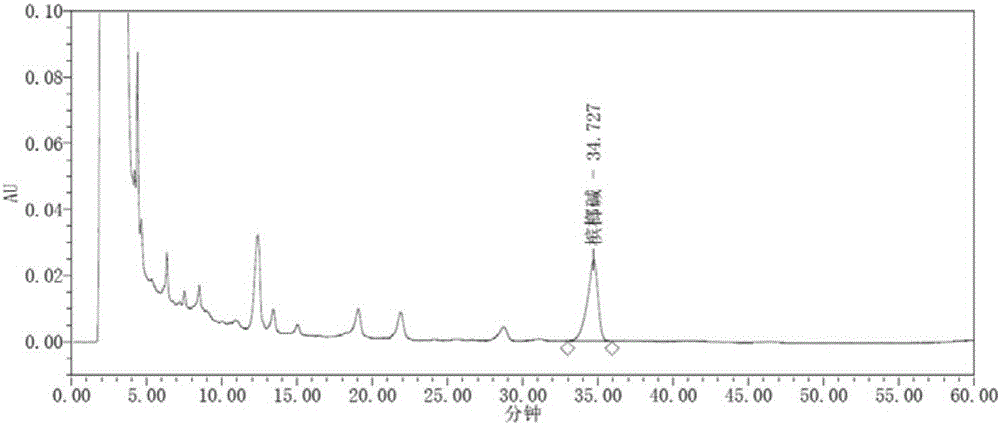
[0094] Embodiment 2. HPLC method is determined the content of arecoline in the composition of Dayuan drink
[0095] (1) Chromatographic conditions
[0096] Use strong cation exchange bonded silica gel as filler, chromatographic column: Phenomenex Luna SCX, 4.6*250mm, 5um, use acetonitrile-phosphoric acid solution (2→1000, adjust the pH value to 3.8 with concentrated ammonia test solution) (50:50) as Mobile phase; the detection wavelength is 215nm, the flow rate is 1.0ml / min, and the column temperature is 30°C. The number of theoretical plates should not be less than 3000 based on the peak of arecoline.
[0097] (2) Preparation of reference substance solution
[0098] Take an appropriate amount of arecoline hydrobromide, weigh it accurately, add acetonitrile-0.2% phosphoric acid solution (2→1000, adjust the pH value to about 3.8 with concentrated ammonia test solution) as the mobile phase (50:50) to make a solution containing 40 μg per 1 ml Solution, that is. (arecoline w...
Embodiment 3
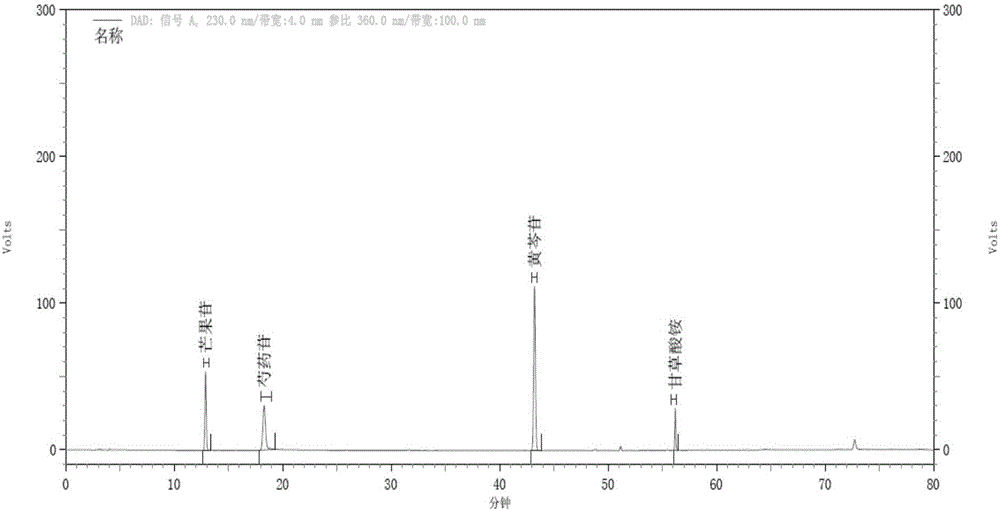
[0108] Example 3. HPLC Determination of Mangiferin, Paeoniflorin, Baicalin and Glycyrrhizic Acid Contents in Dayuan Decoction Composition
[0109] (1) Chromatographic conditions: Octadecylsilane bonded silica gel as filler, chromatographic column: Agilent ZORBAX Eclipse Plus C18, 4.6*250mm, 5μm, acetonitrile as mobile phase A, 0.2% phosphoric acid solution as mobile phase B, Perform gradient elution as specified in the table below; flow rate: 0.8ml / min, column temperature: 30°C. The number of theoretical plates calculated based on the baicalin peak should not be less than 3000.
[0110] Mobile Phase Gradient Program
[0111]
[0112] Detection wavelength table
[0113]
[0114] (2) Preparation of reference substance solution
[0115] Take the appropriate amount of mangiferin reference substance, paeoniflorin reference substance, baicalin reference substance, and ammonium glycyrrhizinate reference substance, accurately weigh them, and add 70% methanol to make each 1m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com