High performance liquid chromatography analysis method of sirolimus
A high-performance liquid chromatography and analysis method technology, applied in the field of drug analysis, can solve the problems of poor stability of experimental results and large data deviation, and achieve the effects of good stability and strong data reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
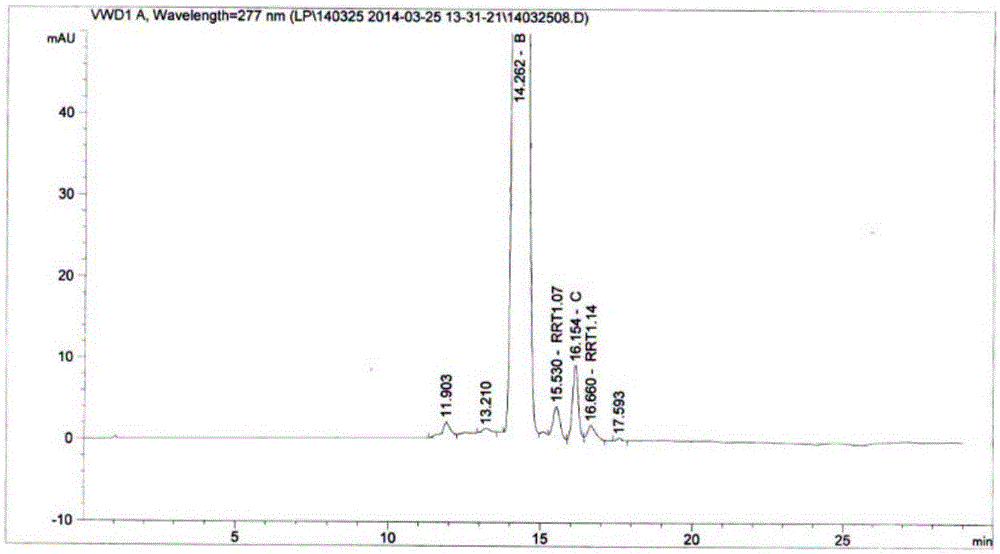
[0026] Determination of sirolimus raw material content and impurities.
[0027] High performance liquid chromatography conditions:
[0028] Chromatographic column: Zorbax Eclipseplus C18, size 4.6mm×150mm, 3μm;
[0029] Mobile phase ratio is: mobile phase A: mobile phase B = 20:80 (volume ratio), mobile phase A is composed of phosphoric acid: sodium heptanesulfonate: water = 0.1:0.01:1 (mass ratio), mobile phase Consists of methanol: acetonitrile=15:85 (volume ratio) in B;
[0030] The detection wavelength is 277nm;
[0031] The flow rate is 1.5ml / min;
[0032] Column temperature 30°C;
[0033] Injection volume 20μL;
[0034] Diluent: 0.1% phosphoric acid solution: acetonitrile = 50:70 (volume ratio).
[0035] Experimental steps:
[0036] Solution preparation:
[0037] Take about 25 mg of sirolimus raw material, weigh it accurately, place it in a 25ml volumetric flask, add an appropriate amount of diluent to dissolve and dilute to the mark, shake well, and use it as th...
Embodiment 2
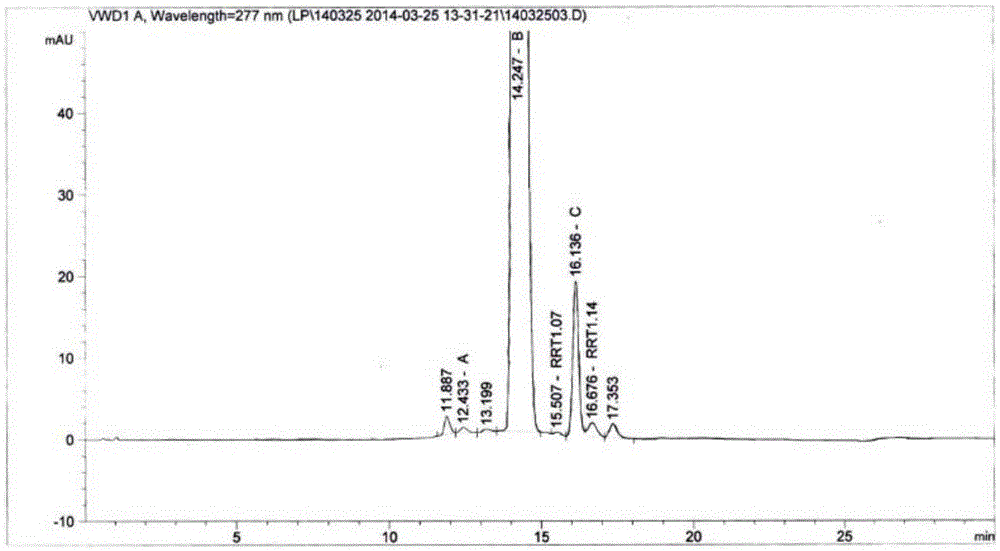
[0044] Determination of content and impurities in sirolimus tablets.
[0045] High performance liquid chromatography conditions:
[0046] Chromatographic column: Shimadzu InertsilC18, size 4.6mm×150mm, 3μm;
[0047] Mobile phase ratio is: mobile phase A: mobile phase B = 18:82 (volume ratio), mobile phase A is composed of phosphoric acid: sodium heptanesulfonate: water = 0.1:0.01:1 (mass ratio), mobile phase B is made up of methanol: acetonitrile=15:85 (volume ratio);
[0048] The detection wavelength is 277nm;
[0049] The flow rate is 1.6ml / min;
[0050] Column temperature 25°C;
[0051] The injection volume was 20 μL.
[0052] Diluent: 0.1% phosphoric acid solution: acetonitrile = 50:75 (volume ratio).
[0053] Experimental steps:
[0054] Solution preparation:
[0055] Take an appropriate amount of sirolimus tablet (approximately equivalent to 25 mg sirolimus), weigh it accurately, place it in a 25ml volumetric flask, add an appropriate amount of diluent to dilute ...
Embodiment 3
[0062] Determination of content and impurities in sirolimus capsules.
[0063] High performance liquid chromatography conditions:
[0064] Chromatographic column: Dima II C18, the size is 4.6mm×150mm, 3μm;
[0065] Mobile phase ratio is: mobile phase A: mobile phase B = 22:78 (volume ratio), mobile phase A is composed of phosphoric acid: sodium heptanesulfonate: water = 0.1:0.01:1 (mass ratio), mobile phase B is made up of methanol: acetonitrile=15:85 (volume ratio);
[0066] The detection wavelength is 277nm;
[0067] The flow rate is 1.8ml / min;
[0068] Column temperature 28°C;
[0069] The injection volume was 20 μL.
[0070] Diluent: 0.1% phosphoric acid solution: acetonitrile = 50:65 (volume ratio).
[0071] Experimental steps:
[0072] Solution preparation:
[0073] Take an appropriate amount of sirolimus capsule content (approximately equivalent to 25 mg sirolimus), weigh it accurately, place it in a 25ml volumetric flask, add an appropriate amount of diluent to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com