Quality detection method for fingerprint spectrum method of radix astragali saponin injection
A quality detection method and fingerprint technology, which are applied in the field of injection quality detection, can solve the problems of not involving the fingerprint of astragaloside injection, few studies, etc., and achieve the effects of good consistency, good reproducibility and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0015] Experimental Example 1 Determination of Common Peaks in Fingerprint of Astragaloside Injection
[0016] 1. Fingerprint
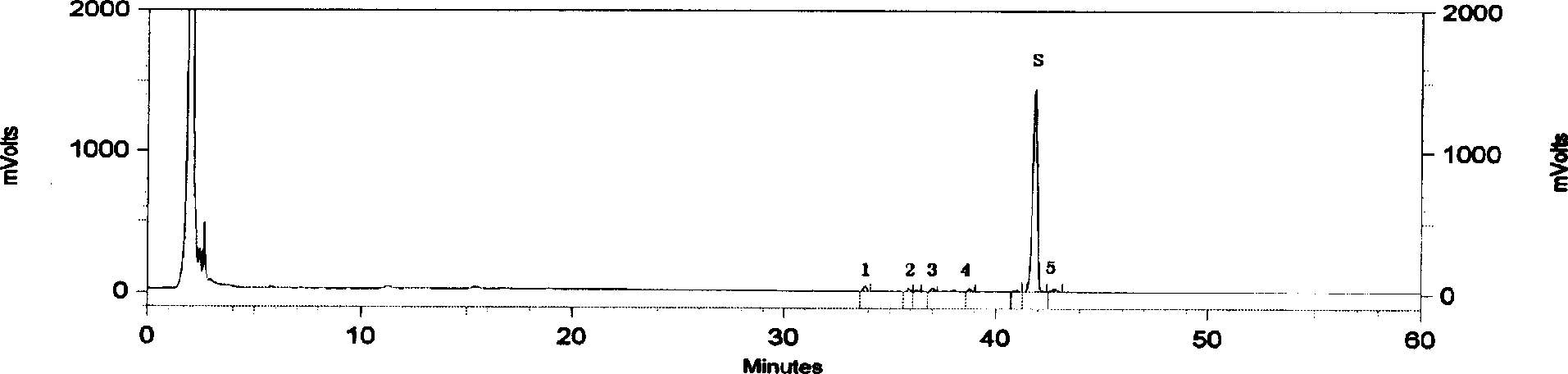
[0017] According to the relevant parameters given by 3 batches of test sample HPLC chromatograms, the main chromatographic peaks of astragaloside injection measured by the aforementioned preparation method all appeared within 60 minutes; Chromatographic peaks did not appear. Comparing the chromatographic peaks of each batch of samples, it was found that 6 peaks were common to each batch, thus establishing a standard fingerprint.
[0018] 2. Calibration of common fingerprint peaks
[0019] According to the calculation results of the relevant data of the fingerprints of the test products, the average relative retention time (peak number) of each common peak is 0.800 (1), 0.850 (2), 0.873 (3), 0.908 (4), 1.000 (S) ), 1.031(5), as the basis for the common fingerprint peak calibration, the allowable relative deviation is ±10%.
[0020] 3. Relative rete...
experiment example 2
[0031] Experimental example 2 The common peaks of the fingerprint of astragaloside for injection are confirmed
[0032] 1. Fingerprint
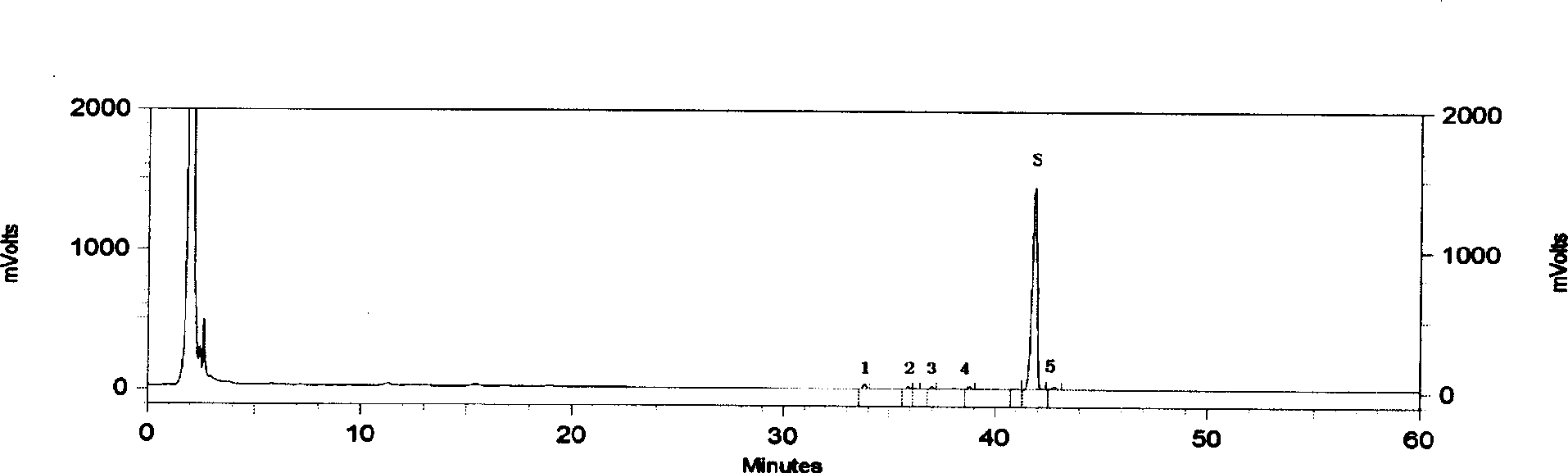
[0033] According to the relevant parameters given by 3 batches of test sample HPLC chromatograms, the main chromatographic peaks of astragaloside for injection measured by the aforementioned preparation method all appeared within 60 minutes; Chromatographic peaks did not appear. Comparing the chromatographic peaks of each batch of samples, it was found that 6 peaks were common to each batch, thus establishing a standard fingerprint.
[0034] 2. Calibration of common fingerprint peaks
[0035] According to the calculation result of relevant data of 3 batches of test product fingerprints, each common peak average relative retention time (peak number) is successively 0.807 (1), 0.857 (2), 0.883 (3), 0.925 (4), 1.000 (S ), 1.023(5), as the basis for the common fingerprint peak calibration, the allowable relative deviation is ±10%.
[0036] 3....
experiment example 3
[0047] Experimental example 3 Astragaloside injection test product solution stability test
[0048] Get astragaloside injection need testing solution of the present invention as measuring object, measure according to aforementioned chromatographic conditions (4 ℃ preserve) respectively at 0 hour, 2 hours, 6 hours, 12 hours, 24 hours, record each common chromatographic peak retention time and Peak area. Taking the retention time of the reference substance astragaloside IV as a reference, the relative retention time of each common peak was calculated, and the results are shown in Tables 7-8.
[0049] Common peak number
[0050] Experiment time
[0051] Experimental result shows: the relative standard deviation of each common peak relative retention time is all less than 1%, very stable; The relative standard deviation of the peak area percentage of reference peak is 0.25%, and the result shows that need testing solution basically keeps stable in 24 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com