Detection method for flupenthixol and melitracen compound drug impurities, new identifiable impurities and safer compound drug
A flupentixol and detection method technology, which can be used in measurement devices, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as unclear source attribution and unknown impurity control amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Verification of impurities:
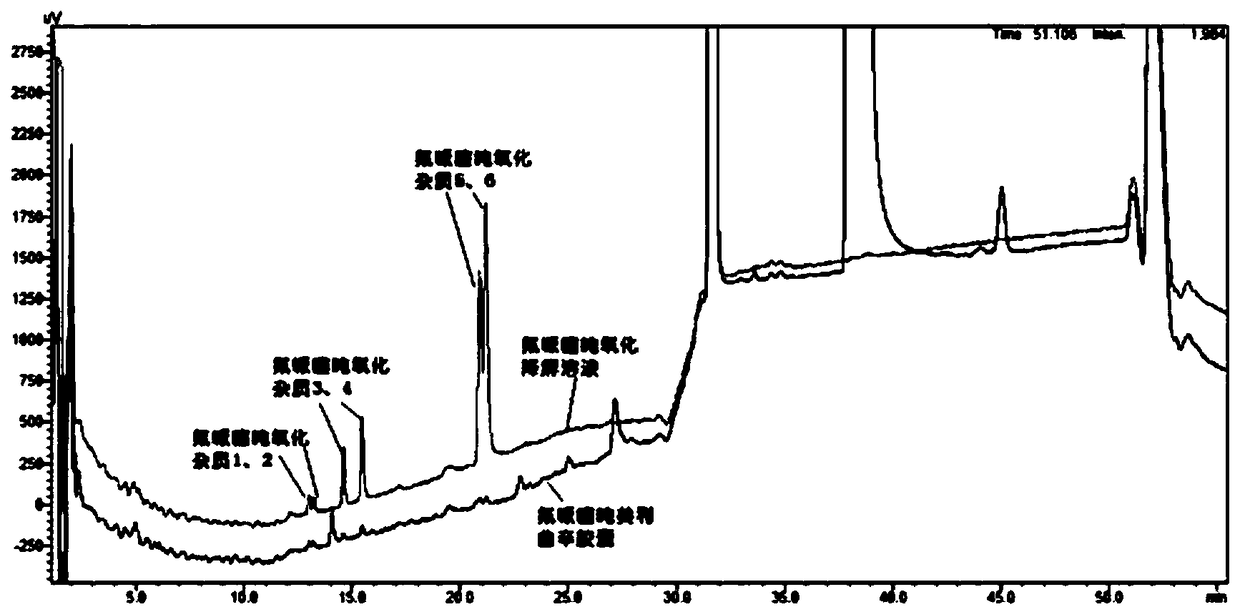
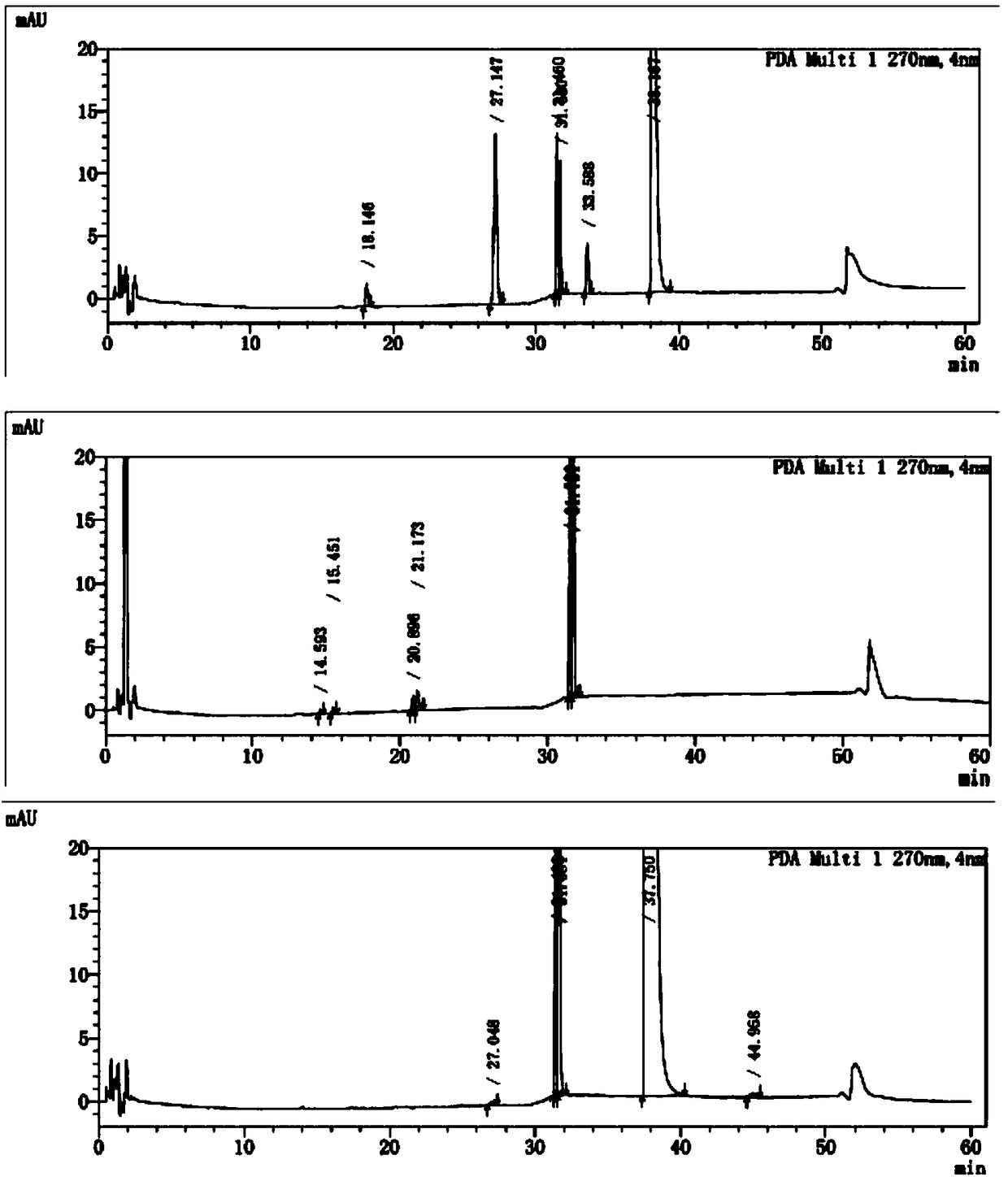
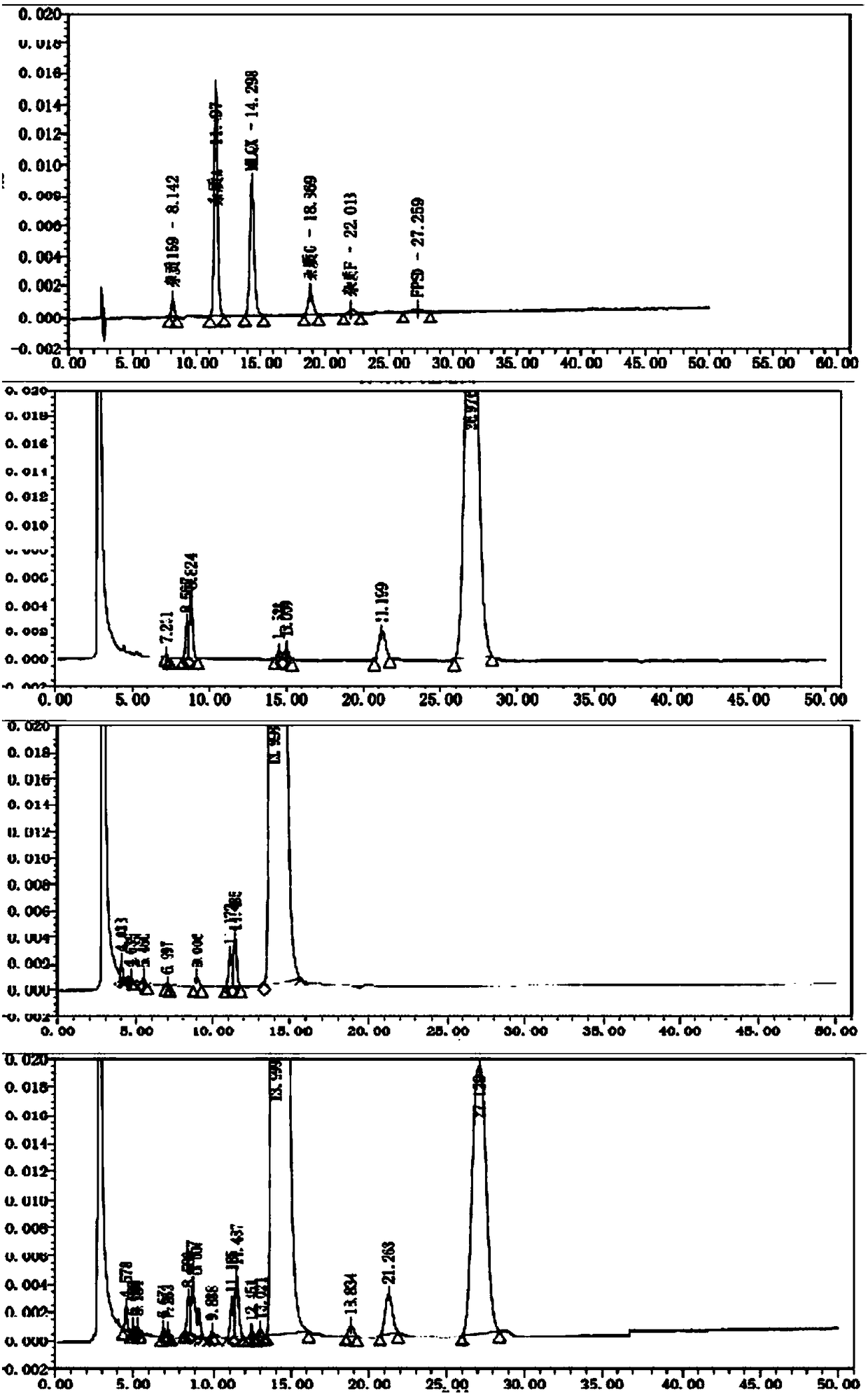
[0082] Take blank excipients, flupenthixol hydrochloride, melitracen hydrochloride, flupentixol hydrochloride plus blank excipients, melitracen hydrochloride plus blank excipients, flupentixol hydrochloride plus melitracen hydrochloride, flupenthixine hydrochloride Tonmelitracen Capsules (hereinafter referred to as the test product) was subjected to drug degradation test; and the degraded sample was analyzed by high performance liquid chromatography, and its chromatogram was recorded; other spectra except the test product were superimposed , and compared with the spectrum measured by the test product, as attached figure 1 Shown, by comparing the same retention time chromatographic peak and its full spectrum in different solution chromatograms, it can be seen that in the test sample, impurities are derived from flupenthixol except known impurity G, impurity Lu-28-159, There are also 6 unknown impurities, which are consistent with the substa...
Embodiment 2
[0085] Detect and calculate the impurities and their content in flupentixol melitracen compound drug according to the following process:
[0086] (1) Take the standard reference substances of impurity A and impurity G, accurately weigh them, dilute and dissolve with methanol to constant volume, and make impurity stock solutions containing about 0.2 mg of impurity A and 0.1 mg of impurity G in every 1 mL of the mixed solution;
[0087] (2) Take flupentixol hydrochloride standard reference substance, melitracen hydrochloride standard reference substance, impurity Lu-28-159 standard reference substance (during actual operation, use the salt Lu 28-159-HCl of impurity Lu-28-159 For the injection object), accurately weighed and dissolved with an appropriate amount of diluent, the diluent includes mobile phase A solution and methanol with a concentration of 0.01mol / L, and the volume ratio of the two is 35:65 to obtain the second stock solution;
[0088] (3) Measure the impurity stock...
Embodiment 3
[0145] Embodiment 3, comparative example
[0146] Detect and calculate the impurities and their content in flupentixol melitracen compound drug according to the following process:
[0147] (1) Take the standard reference substances of impurity A and impurity G, accurately weigh them, dilute and dissolve with methanol to constant volume, and make impurity stock solutions containing about 0.2 mg of impurity A and 0.1 mg of impurity G in every 1 mL of the mixed solution;
[0148] (2) Take flupentixol hydrochloride standard reference substance, melitracen hydrochloride standard reference substance, impurity Lu-28-159 standard reference substance (during actual operation, use the salt Lu 28-159-HCl of impurity Lu-28-159 For the injection object), accurately weighed and dissolved with an appropriate amount of diluent, the diluent includes mobile phase A solution and methanol with a concentration of 0.01mol / L, and the volume ratio of the two is 35:65 to obtain the second stock soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com