Method for separating relevant substances of hydrochloric acid palonosetron injection by virtue of reversion phase chromatography
A palonosetron and separation method technology, which is applied in the field of reversed-phase high-performance liquid chromatography for separation of related substances in palonosetron hydrochloride injection, can solve the problems of unsuitable practical operation, damage to instruments and chromatographic columns, and failure to achieve a single separation and other problems, to achieve high sensitivity, simple and convenient method, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
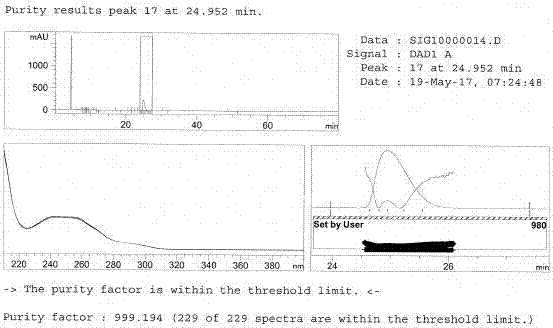
Image
Examples
Embodiment 1
[0025] 1. Instruments and reagents:
[0026] Agilent 1260 liquid chromatograph equipped with G1314F ultraviolet detector and HPLC chromatographic workstation equipped with analytical instruments. Acetonitrile (chromatographic grade), methanol (chromatographic grade), diethylamine (analytical grade), glacial acetic acid (analytical grade), purified water.
[0027] 2. Chromatographic conditions:
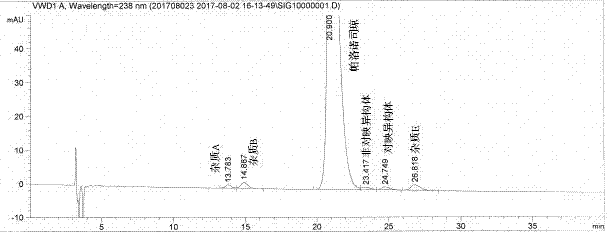
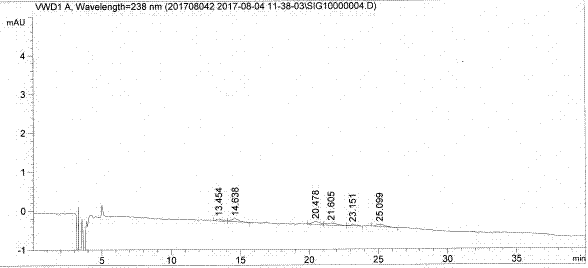
[0028] Chromatographic column: CHIROBIOTIC T column (4.6×250mm, 5.0μm)
[0029] Detection wavelength: 238nm
[0030] Column temperature: 40°C
[0031] Flow rate: 1.0ml / min
[0032] Injection volume: 100μl
[0033] Mobile phase: mobile phase A: mobile phase B=40:60, where mobile phase A is methanol:acetonitrile=90:10, mobile phase B is 0.5% diethylamine aqueous solution (adjust pH to 5.0 with glacial acetic acid).
[0034] Diluent: mobile phase
[0035] 3. Solution Preparation
[0036] To prepare a system suitability solution:
[0037] (1) Impurity A solution: Weigh about 25mg ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com