Method for testing isomer of palonosetron hydrochloride injection solution
A palonosetron and testing method technology, which is applied in the field of testing isomers in palonosetron hydrochloride injection, can solve problems such as difficult analysis, easy superposition, and impact on detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
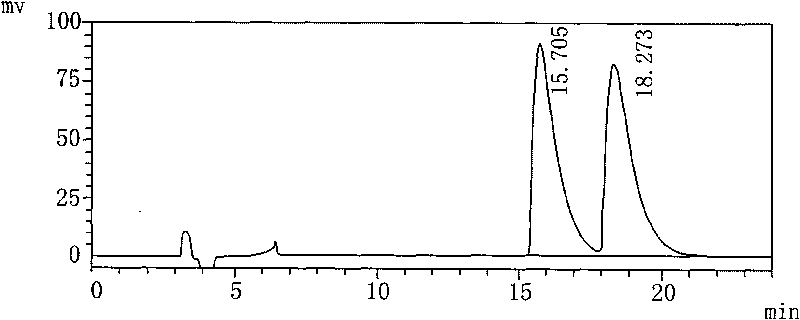
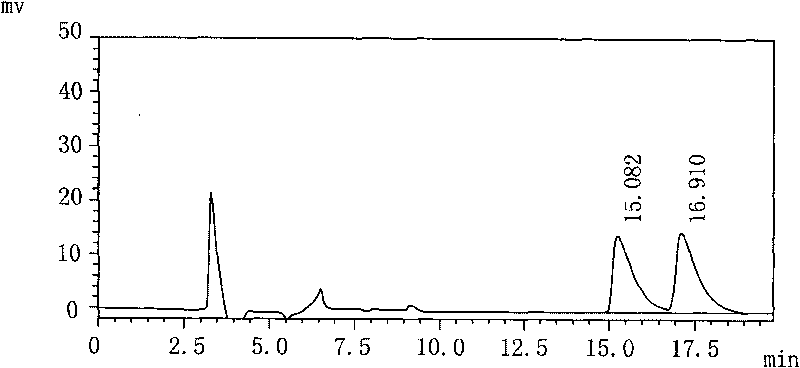
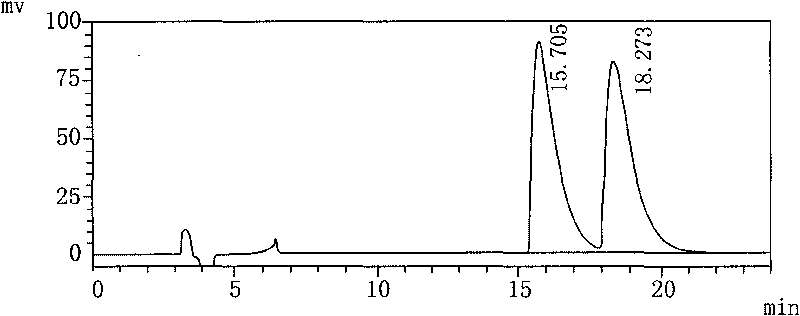
[0017] 1) Preparation of the test solution: Take 100 mL of Palonosetron Hydrochloride Injection, put it in a 250 mL round-bottomed flask, evaporate to dryness under reduced pressure at 48°C, and add 10 mL of isopropanol to the evaporated sample. Ultrasound dissolves the main ingredient, filters, and gets its subsequent filtrate as the test solution;
[0018] 2) Preparation of the refined enantiomer solution: add isopropanol to the refined enantiomer to dissolve, dilute to a solution with a concentration of 0.05 mg / mL, and set aside;
[0019] 3) Mix the upper test solution and the enantiomer refined product solution of equal volume as the mixed pre-test solution;
[0020] 4) The above-mentioned mixed pre-test solution is measured according to high performance liquid chromatography (Chinese Pharmacopoeia 2005 edition two appendix VD). The mixed solution, the volume ratio of the three is n-hexane: Virahol: diethylamine=88: 12: 0.1, the flow rate is 1.0mL / min, the detection wavel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com