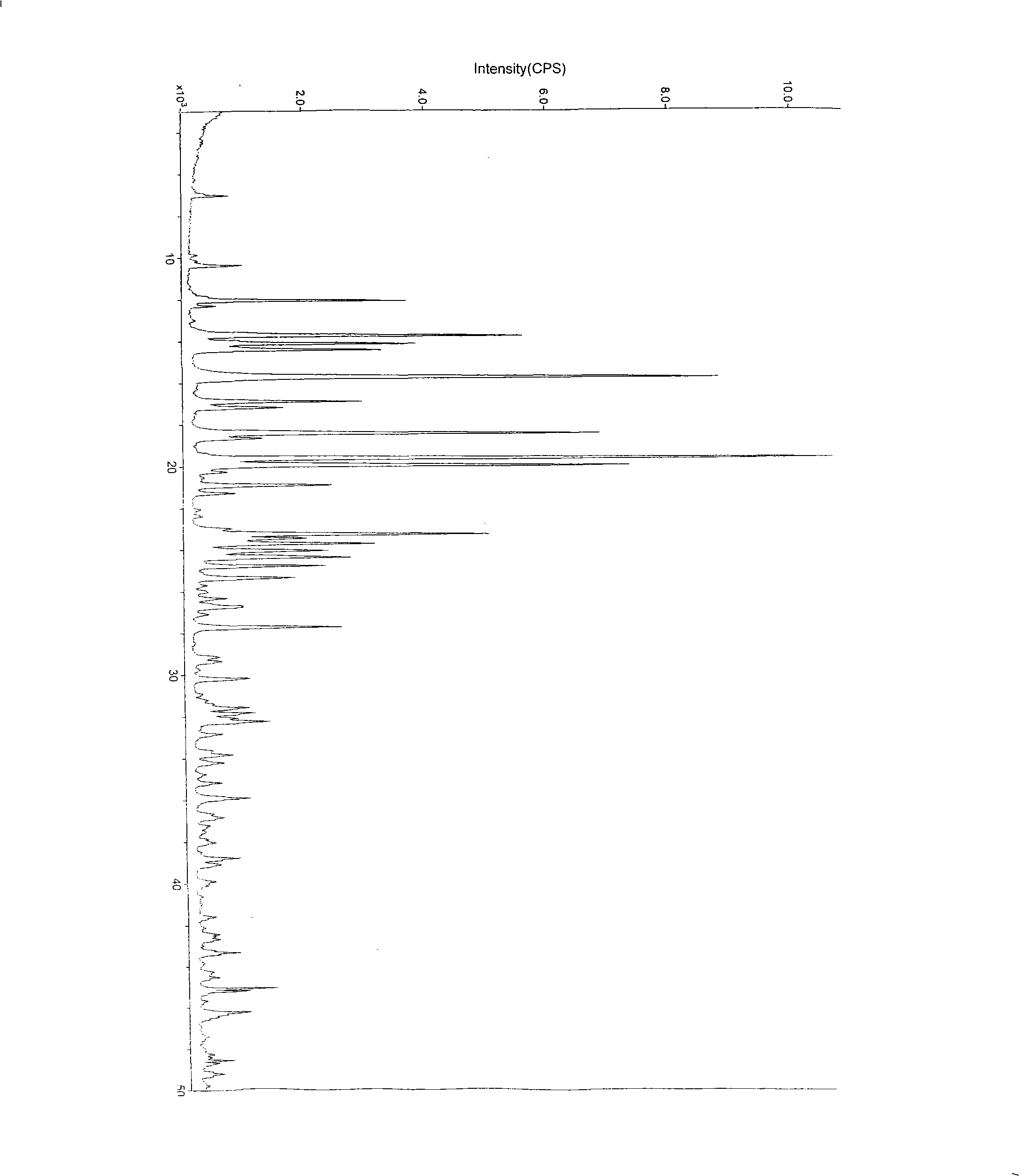
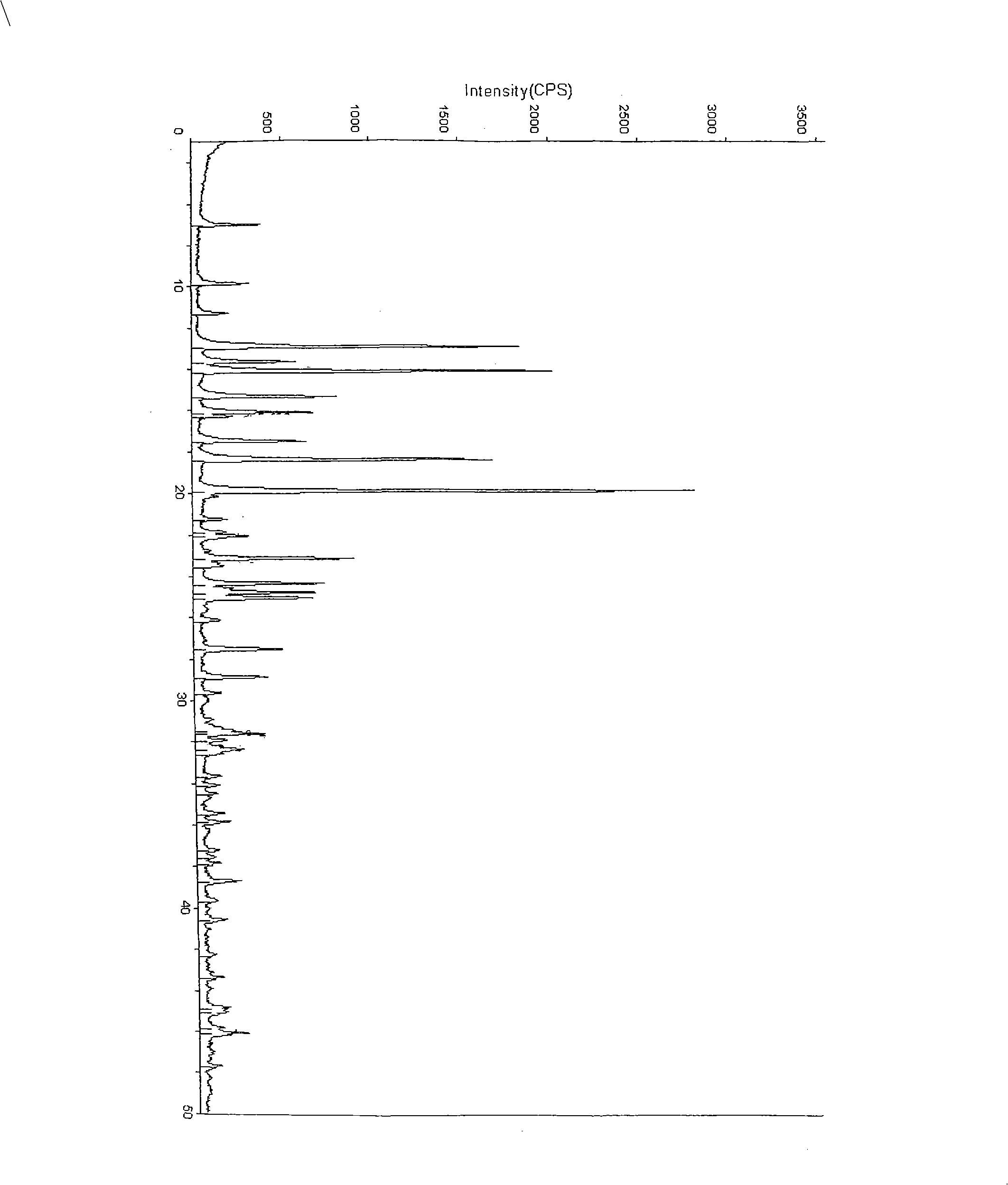
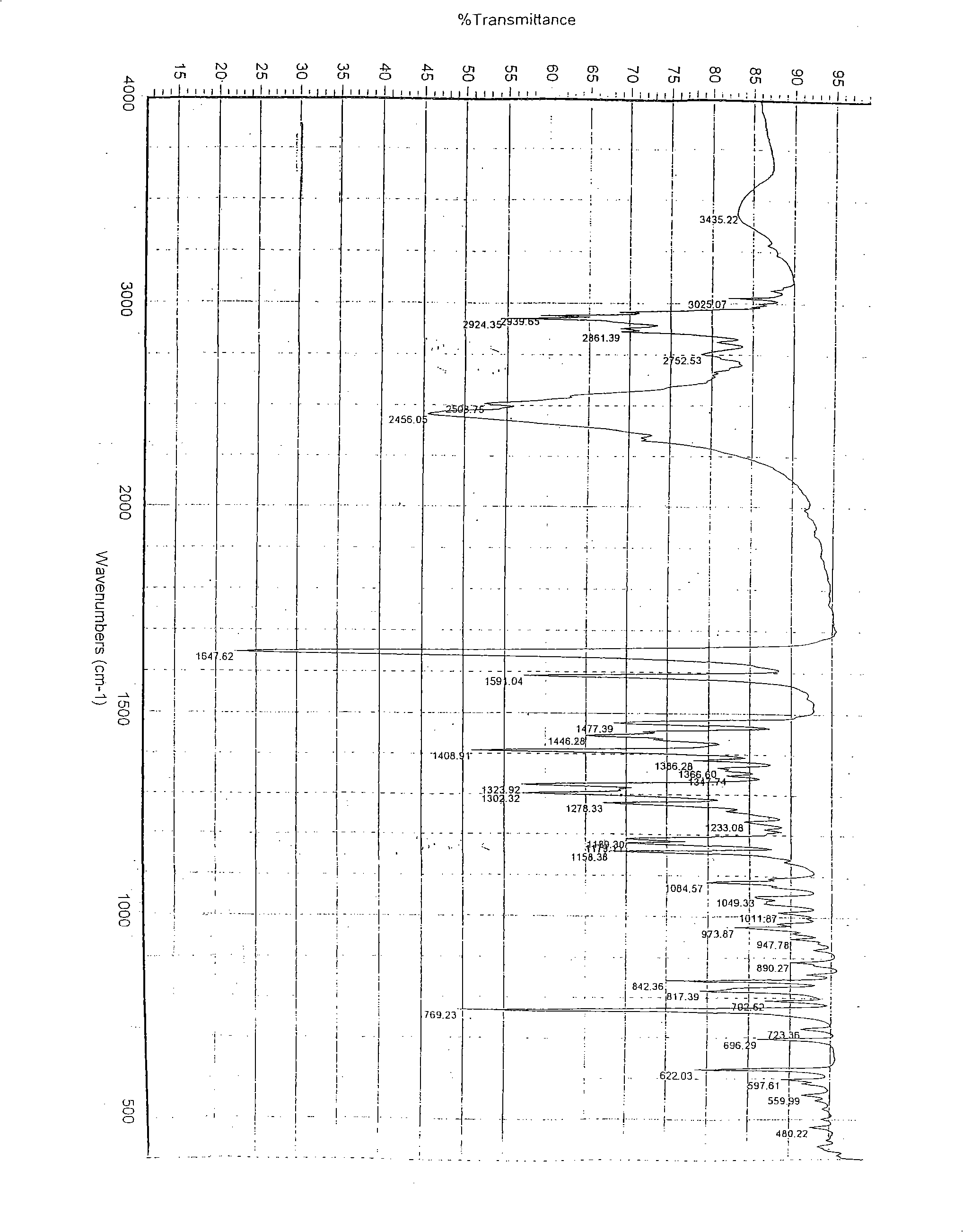
Crystal system of chlorhydric acid palonosetron and preparation method thereof
A technology of palonosetron and hydrochloric acid, which is applied in the field of crystal forms of palonosetron hydrochloride and its preparation, can solve the problems of unmentioned crystal form spectral characteristics, unmentioned palonosetron hydrochloride crystal forms and preparation method etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 palonosetron hydrochloride A crystal form one
[0037]Take 5 g of palonosetron hydrochloride, add 115 ml of isopropanol and 4 ml of water, and heat to dissolve it. After all the solids are dissolved, continue heating, distill 36ml of liquid under normal pressure, cool to 0°C, stir, crystallize for 2 hours, filter after crystallization, and vacuum dry at 80°C for 4 hours to obtain 4.4g of pure crystals. The purity by HPLC was 99.7%.
Embodiment 2
[0038] The preparation of embodiment 2 palonosetron hydrochloride A crystal form two
[0039] Take 8 g of palonosetron hydrochloride, add 124 ml of isopropanol and 6.4 ml of water, and heat to dissolve it. After all the solids were dissolved, 54ml of isopropanol was added, cooled to 0°C, stirred, crystallized for 4 hours, filtered after crystallization, and vacuum-dried at 80°C for 4 hours to obtain 7.56g of pure crystals, the purity of which was determined by HPLC as 99.6%.
Embodiment 3
[0040] The preparation of embodiment 3 palonosetron hydrochloride B crystal form one
[0041] Take 5 g of palonosetron hydrochloride, add 200 ml of ethanol, heat to dissolve the solid, cool, stir and crystallize at 10°C for 2 hours, filter, and dry in vacuum at 80°C for 4 hours to obtain 4.3 grams of pure crystals, which are detected by HPLC The purity is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com