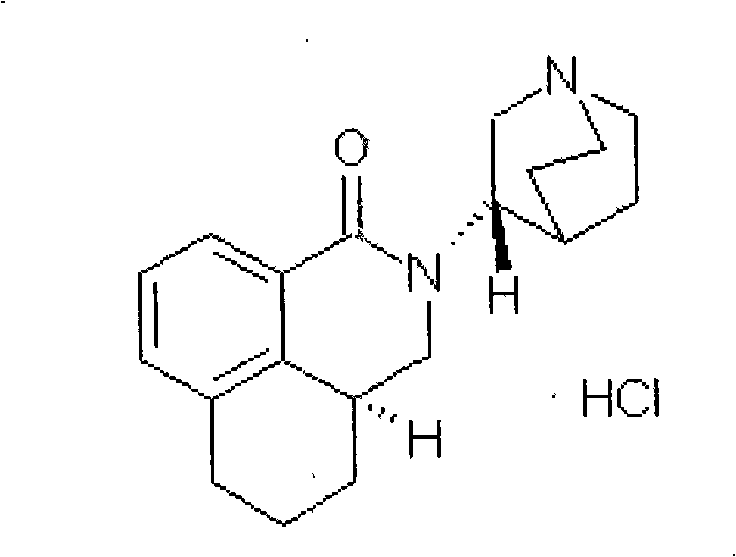
Method for separating and measuring palonosetron hydrochloride optical isomer
A technology of palonosetron and optical isomers, applied in the field of separation and determination of palonosetron hydrochloride optical isomers, to achieve the effect of controlling the internal quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
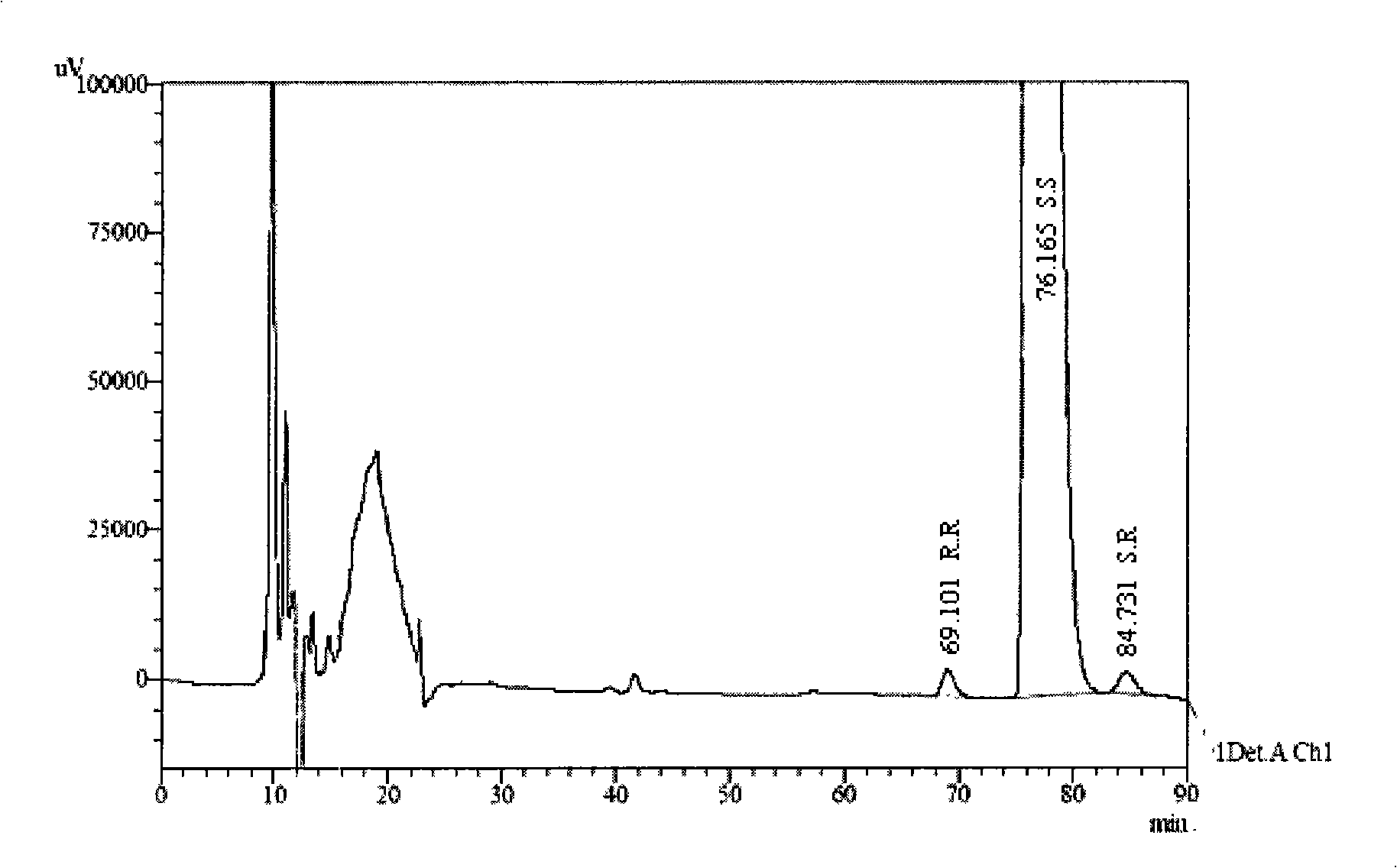
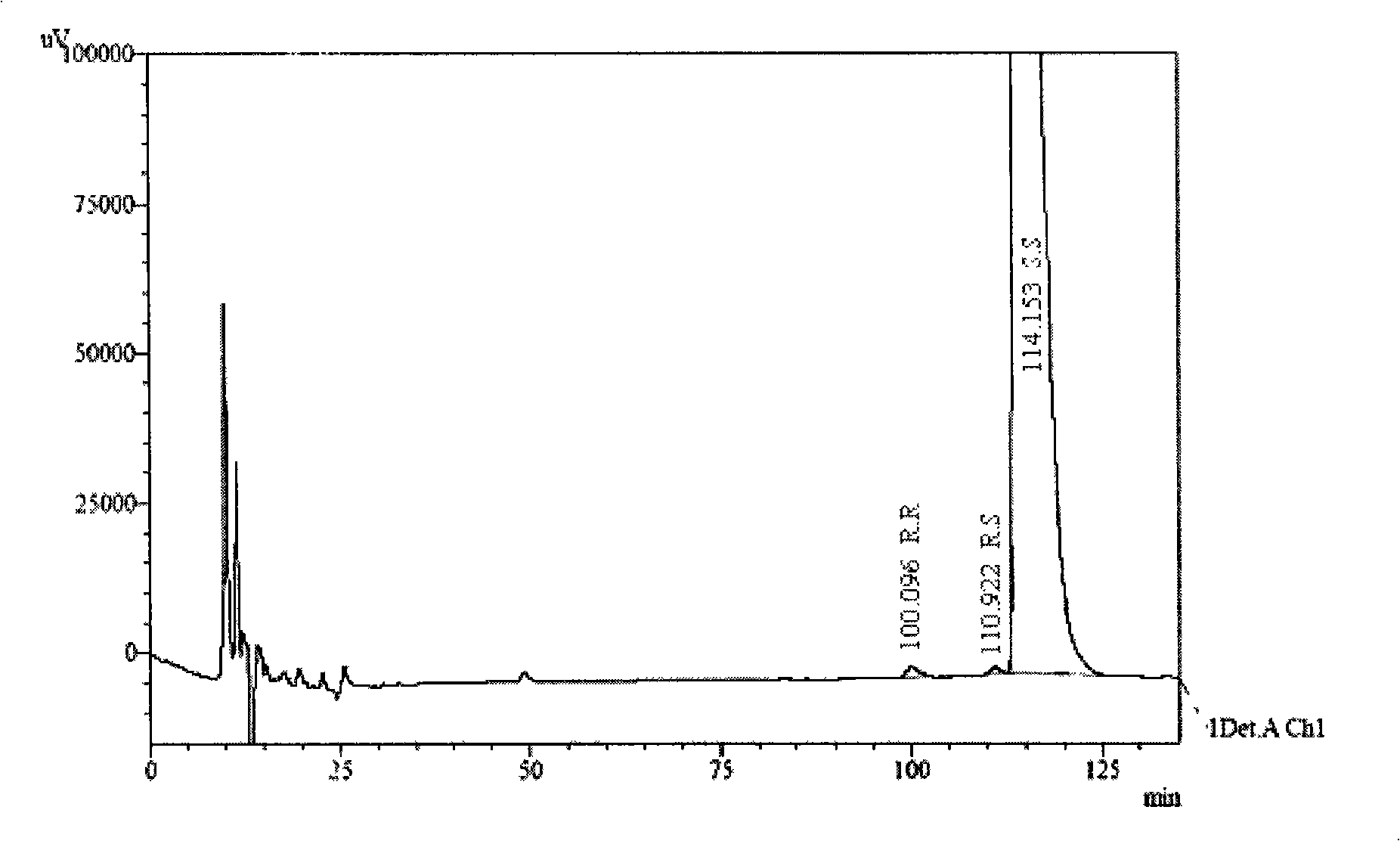
[0016] 1. Instruments and reagents
[0017] 1.1 Instruments and reagents:
[0018] Instrument: Shimadzu 2010 high performance liquid chromatography, chromatographic grade n-hexane, ethanol (Merck), diethylamine (analytical grade).
[0019] Reagents: S.R, R.R, R.S type isomers, three batches of palonosetron hydrochloride raw materials PAL040601, PAL040602, PAL040603 (Shenzhen Wanle Pharmaceutical Co., Ltd.).
[0020] 1.2 Chromatographic conditions:
[0021] Chromatographic column: CHIRALPAK AD-H 5μ250mm×4.6mm (Jiangsu Hanbang Technology Co., Ltd.), with tris(3,5-dimethylphenylcarbamate) amylose as filler
[0022] Detector and detection wavelength: UV-220nm
[0023] Mobile phase: n-hexane: ethanol: diethylamine (92:8:0.4)
[0024] Flow rate: 0.6ml / min, injection volume: 20μl
[0025] Column temperature: 20°C (R.R, R.S, S.S) and 42°C (R.R, S.S, S.R)
[0026] 2. Solution Preparation
[0027] The test solution accurately weighed about 12.5 mg of palonosetron hydrochloride ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com