UPLC characteristic spectrum construction method and application of endothelium corneum gigeriae galli, fried endothelium corneum gigeriae galli and vinegar endothelium corneum
A characteristic map and technology of vinegar gallinaceous gold, applied in the field of analysis and detection, to achieve the effect of ensuring clinical curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The preparation of the test solution is to take the medicinal material of chicken Neijin, the decoction pieces of the chicken, the fried pieces of the chicken, and the decoction pieces of the chicken with vinegar, accurately weigh them, put them in a stoppered conical flask, add methanol, and reflux for 30 minutes. Allow to cool, supplement the lost weight with methanol, shake well, filter, and take the subsequent filtrate.
[0090] Wherein, the ratio of the mass g of the raw materials of the chicken Neijin medicinal material, the chicken Neijin decoction pieces, the fried chicken Neijin decoction pieces, and the vinegared chicken Neijin decoction pieces to the volume mL of the solvent is preferably 1:25.
[0091] Take chicken Neijin standard decoction, stir-fried chicken inner gold standard decoction, vinegar chicken inner gold standard decoction, chicken inner gold formula granules, fried chicken inner gold formula granules, vinegar chicken inner gold formula granules ...
Embodiment 1
[0155] Example 1 Screening of chromatographic conditions
[0156] 1.1 Chromatographic conditions
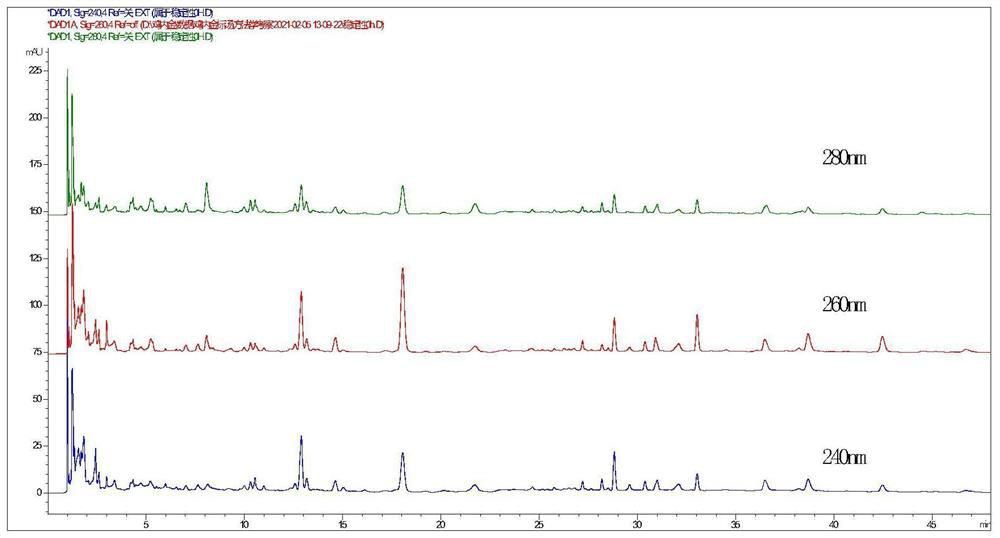
[0157] Use octadecylsilane-bonded silica gel as filler (column length is 150mm, inner diameter is 2.1mm, particle size is 1.6μm); methanol is used as mobile phase A, and 0.02% acetic acid is used as mobile phase B. Gradient elution was specified, and the detection wavelength was 260 nm; the flow rate was 0.3 ml / min, and the column temperature was 40 °C. The number of theoretical plates should not be less than 5000 according to the genistein peak. The mobile phase gradients are shown in Table 1.
[0158] Table 1
[0159]
[0160] 1.2.0 Mobile Phase Selection
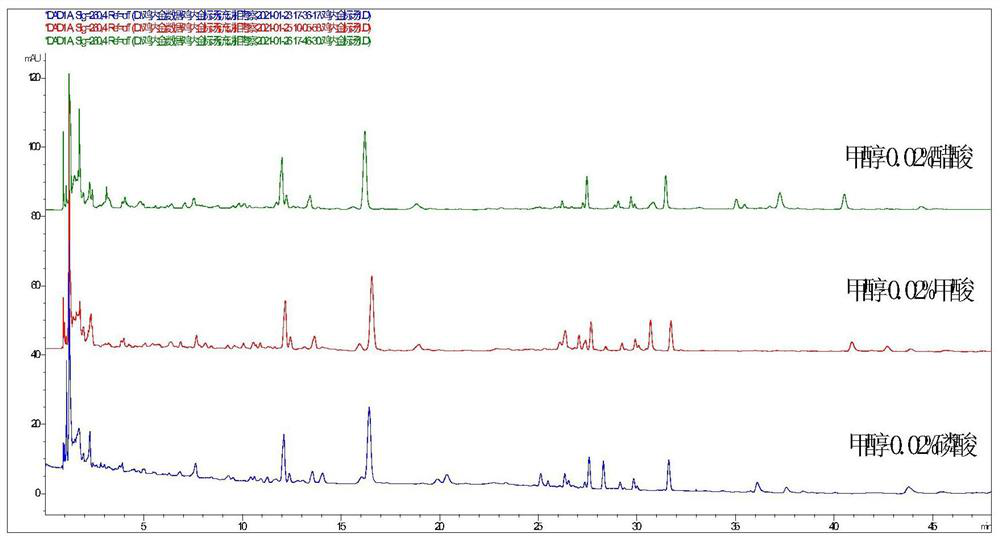
[0161] On the basis of the experimental conditions proposed in 1.1 above, the separation effects of three different mobile phases were investigated: methanol-0.02% acetic acid, methanol-0.02% phosphoric acid, methanol-0.02% formic acid, see figure 1 , figure 1 Select Results Plot for Mobile Phase.
[0162] 1.2.1 Wa...
Embodiment 2
[0202] Example 2 Methodological investigation
[0203] 2.5.1 Chromatographic peak identification
[0204] Preparation of the test solution: According to the experimental conditions proposed above, prepare the chicken Neijin standard decoction for the test solution.
[0205] Preparation of control medicinal material solution: take about 1g of this product, accurately weigh it, add 25ml of water, weigh it, decoct for 30min, let it cool, make up the lost weight with water, shake well, filter, evaporate the filtrate to dryness, add 10ml of methanol , Dense plug, ultrasonic treatment (power 600W, frequency 40kHz) for 30 minutes, let cool, make up the lost weight with methanol, shake well, filter, and take the continuous filtrate.
[0206] Preparation of reference substance solution: Take appropriate amount of genistein, daidzein, genistein, and daidzin reference substance, accurately weigh, and add methanol to make a solution containing 8 μg per 1 ml.
[0207] Preparation of nega...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com