Method for determination of optical isomers in palonosetron hydrochloride composition
A technology of palonosetron and optical isomers, which is applied in the field of medicine and can solve the problems that the accurate determination of impurity content has an influence, and the diastereomers cannot be detected at the same time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Instrument: Shimadzu LC-10AT high performance liquid chromatography system, including LC-10AT pump, SPD-10A ultraviolet detector and HW chromatography workstation.
[0020] Chromatographic column: chiral chromatographic column Chiralpak ADH column (250mm×4.6mm, 5μm).
[0021] Mobile phase: n-hexane-absolute ethanol-diethylamine (93:7:0.2)
[0022] Detection wavelength: 240nm
[0023] Flow rate: 0.6ml / min
[0024] Column temperature: 20°C
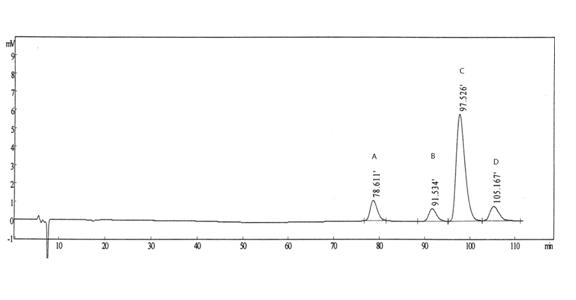
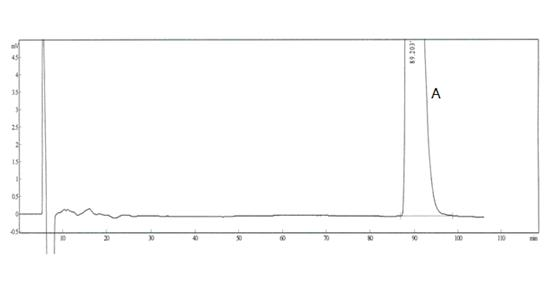
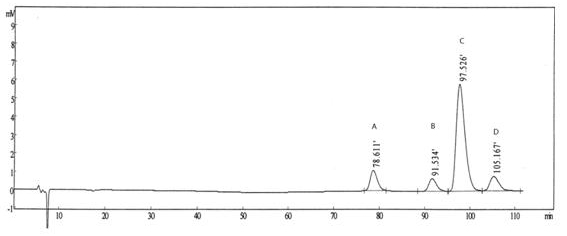
[0025] Reference substance solution: Accurately weigh an appropriate amount of R, R-type palonosetron reference substance, S, R-type palonosetron reference substance and R, S-type palonosetron reference substance, and add mobile phase to quantitatively dilute Prepare a mixed solution containing about 0.5 μg in each 1 ml as a reference solution.
[0026] Need testing solution: take 10 palonosetron hydrochloride compositions, put them in an evaporating dish, concentrate in a water bath to about 5ml, let it cool, add 40ml of ethanol,...
Embodiment 2
[0028] Example 2 Determination of Palonosetron Hydrochloride Composition Preparation Method
[0029] prescription:
[0030] Palonosetron Hydrochloride 0.28g
[0031] Mannitol 207.5g
[0032] Sodium edetate 0.5g
[0033] Citric acid 25g
[0034] Water for injection 5000ml
[0035] Made 1000 pieces
[0036] Weigh the prescribed amount of mannitol, edetate sodium and citric acid, add to 96% water for injection, stir to dissolve, add 0.1mol / L sodium hydroxide solution, adjust the pH value to about 5.0 (4.5-5.5), Add 0.1% activated carbon for needles, stir at 80°C for 30 minutes, decarbonize with a titanium alloy rod while it is hot, then add the prescribed amount of palonosetron hydrochloride, stir to dissolve, make up to the full amount with water for injection, and then inject 0.22μm Filter through a microporous membrane until clear, measure the intermediate content and pH value, and fill it in ampoules with 5ml per bottle, seal it, sterilize it at 121°C for 15 minutes...
Embodiment 3
[0038] According to Example 2, three batches of palonosetron hydrochloride composition samples were prepared and the content of palonosetron hydrochloride isomers in each batch of compositions was detected. The specific results are shown in Table 1.
[0039] Table 1 Inspection results of palonosetron hydrochloride isomers in the palonosetron hydrochloride composition
[0040] Batch number RR isomer RS type isomer SR isomer 100501 not detected not detected not detected 100502 not detected not detected not detected 100503 not detected not detected not detected
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com